1. What are metals? Classify engineering materials.
✔ Metals are elemental substances. Metals are composed of elements which readily give up electrons to provide a metallic bond and electrical conductivity.
✔ Types of metals: 1. Ferrous metals, and 2. Non-ferrous metals.
2. What are ferrous metals? Classify ferrous materials.
✔ The metals, which contain iron as their main constituent, are called ferrous metals.
✔ Types of ferrous metals: 1. Steels, and 2. Cast irons.
3. State three reasons why ferrous alloys are used extensively.
1. Iron-based components are relatively abundant and are widely distributed throughout the world.
2. Ferrous materials can be produced very economically.
3. Ferrous materials are versatile. Therefore wide range of mechanical and physical properties of ferrous materials can be achieved.
4. State three characteristics of ferrous alloys that limit their utilization.
Heavy in weight, Lower electrical and thermal conductivity, lower resistance to corrosion.
5. How can you specify a steel? What is the difference between 4140 steel and 4340 steel?
✔ The AISI/SAE designation for the steels is a four digit number: First two digits indicate the alloy content, and Last two digits indicate the carbon concentration.
✔ 4140 steels is alloy of Cr-Mo with 0.40% C, whereas 4340 steel is an alloy of Mo-Cr-Ni with 0.40% C.
6. What are the three primary groups of plain carbon steels?
1. Low-carbon steels: Those contain less than 0.25% carbon.
2. Medium-carbon steels: Those containing between 0.25 and 0.60% carbon.
3. High-carbon steels: Those containing more than 0.60% carbon.
7. What are alloy steels? How are alloy steels classified?
✔ Alloy steels mean may steels other than carbon steels.
✔ Alloy steels can be divided into two main groups as:
1. Low alloy steels: These contain upto 3 to 4% of alloying elements.
2. High alloy steels: These contain more than 5% of alloying elements.
8. List four important alloying elements added in alloy steels.
The most commonly used alloying elements are chromium, nickel, molybdenum, vanadium, tungsten, cobalt, boron, copper and others.
9. Why is alloying done?
The alloying of steel is generally done:
✔To increase its strength.
✔ To improve hardness.
✓ To improve toughness.
✔ To improve resistance to abrasion and water.
✔ To improve machinability.
✔ To improve ductility.
10. What are the primary effects of chromium, and copper as alloying elements in steel?
✔ Effects of alloying chromium: Increases corrosion and oxidation resistance, increases hardenability, increases high-temperature strength, and resists abrasion and wear (with high carbon).
✔ Effects of alloying copper: Increases strength, and increases corrosion resistance.
11. What are the effects of lead and sulphur on the machinability of steels?
Lead improves the machinability whereas sulphur reduces it.
12. Which alloy elements are basically (a) carbide formers, and (b) graphite promoters?
(a) Carbide formers: Cr, W, Ti, Mo, Nb, V, and Mn.
(b) Graphite promoter: Si, Co, Al, and Ni.
13. What makes a stainless steel "stainless"?
The chromium oxide (extremely dense-thin) protective layer acts as a barrier to retard further oxidation, rust or corrosion. As this steel cannot be stained easily, it is called stainless steel.
14. Why do stainless steels lose their corrosion resistance when the chromium in solution drops below 12%?
When the weight% of chromium drops below 12% the corrosion rate increases sharply. As the corrosion rate increases, the resultant chromium-oxide protective layer unable to retard oxidation, rust or corrosion effectively.
15. What determines whether a stainless steel is austenitic, ferritic, or martenistic?
The predominant phase constituent of the microstructure present in a stainless steel determines whether a stainless steel is austenitic, ferritic, or martenistic.
16. What are the required properties of a tool steel?
Tool steels should have the following requirements:
(i) Good toughness.
(ii) Good wear resistance.
(iii) Very good machinability.
(iv) Slight change of form during hardening.
(v) Little risk of cracking during hardening.
(vi) Resistance to softening on heating.
17. How can you classify tool steels?
1. Cold work tool steels
2. Shock resisting tool steels,
3. Hot work tool steels,
4. High speed tool steels,
5. Plastic mold tool steels, and
6. Special purpose tool steels.
18. What is meant by 18-4-1 high speed steel?
A widely used high-speed tool steel is 18-4-1 high speed steel. This steel contains 18% tungsten, 4% chromium, and 1% vanadium. It is considered to be one of the best of all purpose tool steels.
19. What are HSLA steels? Where are they used?
✓ HSLA steels are nothing but high-strength low-alloy steels. HSLA steels, also known as micro alloyed steels, are low-carbon steels containing small amounts of alloying elements.
✓ These HSLA steels are widely used as structural or constructional alloy steels.
20. What are maraging steels? Give its composition.
✓ Maraging steels are low-carbon, highly alloyed steels. These are very high-strength materials that can be hardened to obtain tensile strengths of upto 1900 MPa.
✓ Composition: Maraging steels contain 18% nickel, 7% cobalt, and small amounts of other elements such as titanium. The carbon content is low, generally less than 0.05%.
21. What are the heat resisting steels and free-machining steels?
✓ Steels which can resist the creep and oxidation at high temperatures and retain sufficient strength are called heat resisting steels.
✓ Free-machining steels, also known as free cutting steels, machine readily and form small chips so as to reduce the rubbing against the cutting tool and associated friction and wear.
22. What are the features that make cast iron an important material?
1. It is a cheap metallurgical substance.
2. Good mechanical rigidity and good strength under compression.
3. Easy castability.
4. Good machinability can be achieved when a suitable composition is selected.
23. What are the effects of carbon on the properties of cast iron?
If a cast iron contains more of the brittle cementite, then its mechanical properties will be poor.
24. What is the influence of cooling rate on the properties of a cast iron?
High rate of cooling results in a weak and brittle cast iron. Slow cooling rate results in tough and strong cast iron.
25. How can you classify cast irons?
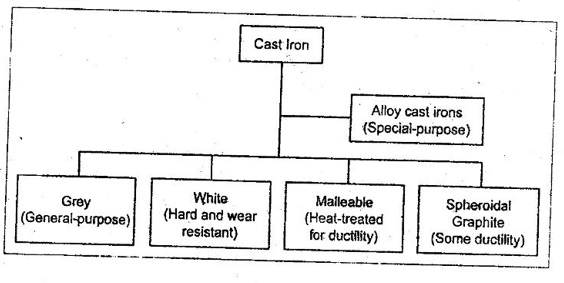
26. What is the chemical composition of grey cast iron?
Typical composition of grey cast iron is given below:
Carbon - 2.5 to 4%
Silicon - 1 to 3%
Manganese - 0.4 to 1%
Phosphorus - 0.15 to 1%
Sulphur - 0.02 to 0.15%
Remaining is iron
27. Where are white cast irons used?
1. White cast iron is used as a raw material in the production of malleable cast iron.
2. The typical applications of white cast irons include rolls, wear plates, pump linings, balls, etc.
3. It is also used for inferior casting and in places where hard coating is required as in the outer surface of car wheels.
28. What is the difference between malleable cast iron and ductile cast iron?
✓ Malleable cast iron is produced by heat treating unalloyed white iron. The ductile (or SG or nodular) cast iron is produced by adding magnesium and/or cerium to molten cast iron.
✓ Both malleable and ductile cast irons have the nodules, also called spheroids. But the nodules of ductile cast irons are more perfect spheres.
29. What are alloy cast irons?
✓ The alloy cast irons, like alloy steels, can be produced by adding alloying elements like Ni, Cr, Mo, Cu, Si, and Mn.
✓ Alloy cast irons have been produced to give high-strength materials, hard and abrasion-resistant materials, corrosion resistant irons, and irons for high-temperature service.
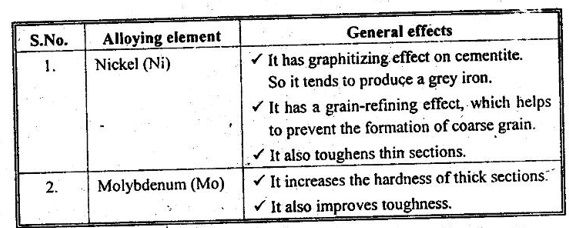
30. What are the primary effects of adding Ni, and Mo cast irons?
31. What properties do non-ferrous alloys have that usually are not associated with ferrous alloys?
1. Lighter in weight.
2. Higher electrical and thermal conductivity.
3. Better resistance to corrosion.
4. Ease of fabrication (casting, rolling, forging, welding, and machining).
5. Colour.
32. Write some characteristics of non-ferrous alloys that limit their utilization.
Non-ferrous materials are not produced in as great tonnages and are more costly than ferrous materials.
33. List the outstanding properties of copper and some typical applications.
✓ The copper posses the following properties:
1. Copper possess very high electrical conductivity.
2. It also has very high thermal conductivity.
3. It exhibits excellent resistance to corrosion.
4. It is very soft, ductile and malleable.
✓ Copper is extensively used for manufacturing power cables, telephone cables, cables for computer networks, printed circuit boards, connectors, etc.
34. What is the main difference between a brass and a bronze?
Brass is an alloy of copper and zinc whereas bronze is an alloy of copper and tin.
35. List at least four types of brasses used.
Gliding metal (or commercial bronze), cartridge brass, standard brass (or cold working brass), Muntz metal (or yellow metal), Naval brass, Admiralty brass.
36. List some bronze alloys.
Bell bronze, phosphor bronze, aluminium bronze, silicon bronze, coinage bronze, and leaded bronze.
37. What are gun metals? Give its composition.
✓ Gun metals are alloys of copper, tin, and zinc.
✓ Composition of admiralty gun metal: 88 Cu, 10 Sn, 2 Zn, 2 (max) Ni.
38. What are cupronickels? What is the use of monel metal?
✓ Cupronickels are alloys of copper and nickel.
✓ Uses of monel metal: For making propellers, pump fittings, condenser tubes, steam turbine blades, sea water exposed parts, tanks, and chemical and food handling plants.
39. What properties have made aluminium and its alloys the most important non-ferrous metal?
(i) Light-weight (one-third the weight of steel),
(ii) High thermal and electrical conductivity,
(iii) Excellent corrosion resistance,
(iv) Non-toxicity,
(v) Soft and ductile,
(vi) Low specific gravity,
(vii) High strength-to-weight ratio, and
(viii) High reflectivity.
40. Why does the aluminium replace the copper as an electrical conductor?
(i) The price of the aluminium is much lower than that of copper.
(ii) The specific gravity of aluminium is only 2.7 compared to 8.9 for copper.
(iii) The electrical conductivity of EC-grade (electrical conductor) aluminium is 61% of the conductivity of standard copper, based on equal cross sections.
(iv) If equal weights of aluminium and copper conductors of a given length are compared, it is found that aluminium conducts 201% as much current as does copper.
41. What are the two types of aluminium alloys?
1. Heat-treatable aluminium alloys, and
2. Non-heat treatable aluminium alloys.
42. What is duralumin? Give its composition and applications.
✓ Duralumin is an alloy of aluminium and copper.
✓ Composition: 94 Al, 4 Cu, 0.5 Mg, 0.5 Mn, 0.5 Si, 0.5 Fe.
✓ Typical applications: For aircraft and automobile industries; for making electric cables, in surgical and orthopaedic implements or gadgets, etc.
43. What is meant by precipitation hardening?
Precipitation hardening, also known as age hardening, is the most important method of improving the physical properties of some of the non-ferrous alloys by solid state reaction.
44. Differentiate between natural ageing and artificial ageing.
✓ The ageing process done at room temperature is often called natural ageing. Natural ageing takes a prolonged period of time in terms of several days to reach maximum strength.
✓ Ageing at high temperature of 190°C to 260°C accelerates the precipitation process and the time required is reduced considerably. This process is called artificial ageing.
45. What is the effect of ageing temperature and time on the material strength?
The strengthening process accelerates with the increase in the ageing temperature. The maximum strength increases as the ageing temperature decreases.
46. What are the required characteristics of a bearing material?
1. Bearing material should possess sufficient hardness and wear resistance.
2. It should have a low coefficient of friction.
3. It should be tough, shock-resistant, and sufficiently ductile.
4. It should have a sufficient melting point, and high thermal conductivity.
5. It should have good casting qualities, and good resistance to corrosion.
47. List the bearing materials that are commonly used.
1. White metals,
2. Copper-base alloys,
3. Aluminium-base alloys,
4. Plastic materials, and
5. Ceramics.
48. What are super alloys?
✓ Super alloy is a general term used to describe the nickel- base and cobalt-base alloys which have been developed for use at elevated temperatures.
✓ Super alloys produce a combination of high strength at elevated temperature, resistance to creep at temperatures upto 1000°C, and resistance to corrosion.
No comments:
Post a Comment