A boiler is a device which is used to generate high-pressure steam by supplying heat to the water.
FIRST LAW OF THERMODYNAMICS - APPLICATION TO OPEN SYSTEMS
1. Boiler (or) Steam Generator
A boiler is a device which is used to generate high-pressure steam by supplying heat to the water. In this system, the heat energy is stored in the steam. Therefore, the internal energy (U) exists. The flow energy (pV) exits due to movement of water. But, there is no work done by the system. The potential energy (gz) and kinetic energy (C2/2) are very small. So, it can be neglected.
Therefore, z1 = z2, C1 = C2 and W = 0
Applying the above conditions in SFEE, it becomes
Q = (h2 - h1) …. (1.74)
2. Condenser
A condenser (Figure 1.64) is a device used to condense a hot steam into water by using a coolant. The main function of the condenser is to transfer the heat from steam to coolant. In this system, there is no work done, change in kinetic and potential energies (i.e. W = 0, z1 = z2 and C1 = C2).
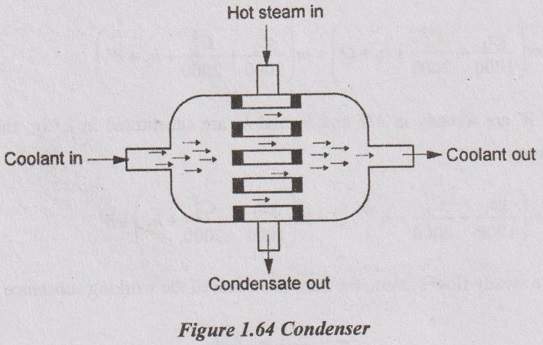
Applying the steady flow energy equation to this system, it can be written as
h1 + Q = h2
Q = h2 - h1 ... (1.75)
3. Nozzle
Nozzle is a device which increases the velocity of kinetic energy of the working substance at the constant pressure drop.
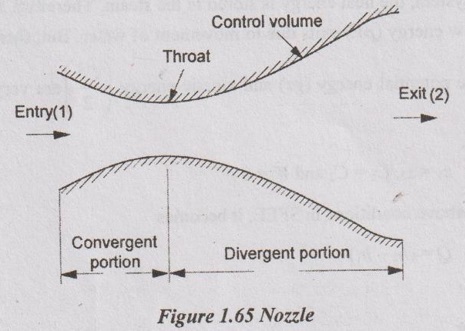
In this system,
(1) There is no work done by the system (W = 0).
(2) There is no heat transfer taking place (Q = 0).
(3) There is no potential energy (z1 = z2).
Applying the steady flow energy equation to this system, it can be written by
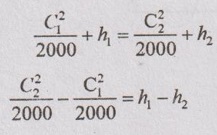
The above equation shows that the increase in kinetic energy will result a decrease in enthalpy.
From above equation, it can be rewritten as
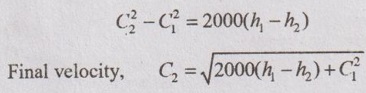
Since, the initial velocity C1 is very small, it can be neglected. Then, the above equation becomes
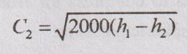
The expansion of the fluid in a nozzle is treated as reversible adiabatic or isentropic.
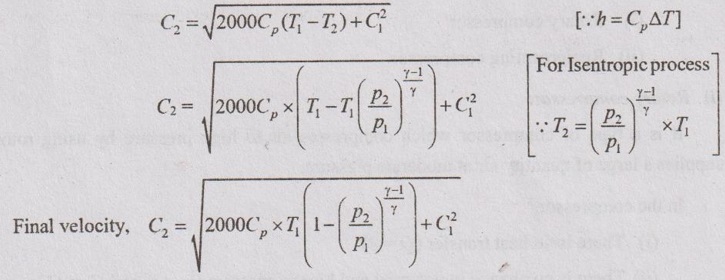
4. Turbine
Turbine is a device which converts the potential energy of working fluid into mechanical work. The turbine is fully insulated. Therefore, there is no heat transfer (Q = 0). In the turbine, the expansion of working fluid is treated as reversible adiabatic or isentropic. The change in potential and kinetic energy is negligible. Therefore, z1 = z2 and C1 = C2.
Applying the SFEE to the above system, it can be written as
h1 = h2 + W
Work output, W = h1 - h2 …. (1.76)
5. Air compressor
Air compressor is a device which is used to compress air from low pressure to high pressure. The input for this compressor is atmospheric pressure.
Compressors are classified into two types:
(i) Rotary compressor
(ii) Reciprocating compressor.
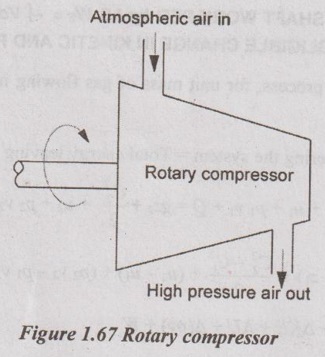
(i) Rotary compressor:
It is a type of compressor which compresses air to high pressure by using rotors. It supplies a large of quantity air at moderate pressure.
In the compressor,
(i) There is no heat transfer (Q = 0)
(ii) There is no change in potential and kinetic energies (z1 = z2 and C1 = C2)
Then, the steady flow energy equation is written as
h1 = h2 - W
A negative sign in - W shows that the work is done on the system.
⸫ W = h2 - h1 ... (1.77)
It shows that the work done increases due to increase in enthalpy.
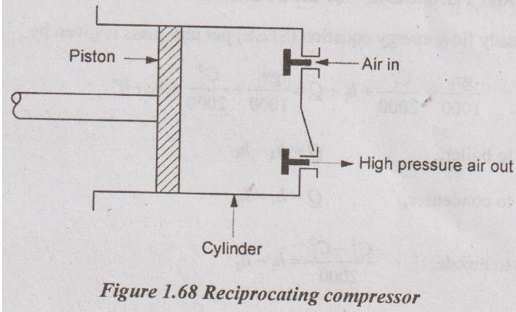
(ii) Reciprocating compressor:
In this type of compressor, the piston reciprocates in the cylinder for producing high- pressure air. It produces very small quantity of air with very high pressure. Larger area is made to contact with the surroundings for producing small quantity of air when compared to rotary compressor. Hence, the heat transfer is more.
In this system, the potential and kinetic energies are negligible (z1 = z2 and C1 = C2)
The steady flow energy equation for this system is expressed by
h1 - Q = h2 - W
Here, negative sign in - W indicates the work is done on the system and - Q indicates the heat rejection to the surroundings.
W = Q + (h2 - h1) ... (1.78)
No comments:
Post a Comment