1. What are polymers?
✓ Polymers are composed of a large number of repeating units of small molecules called monomers.
✓ Polymers may be defined as giant organic, chain-like molecules having molecular weight from 10000 to more than 1,000,000 g.mol-1.
2. List any four attractive characteristics of polymers.
1. Low density.
2. Good thermal and electrical insulation properties.
3. High resistance to chemical attack.
4. Ease of fabrication.
5. Relatively low cost.
3. Classify polymers.
1. Plastics
2. Elastomers
3. Adhesives
4. Coatings
5. Fibres
4. Define the following terms: (i) Monomer, (ii) Homopolymer, and (iii) Copolymer.
Monomer is a small molecule consisting of a single mer i.e., a single unit/blocking block.
Homopolymer is a polymer made out of identical monomer.
Copolymer is a polymer which is obtained by adding different types of monomers.
5. What is meant by isomerism?
Isomerism is a phenomenon wherein different atomic configurations are possible for the same configuration.
6. What is meant by the term 'unsaturated molecule'? State its significance in plastics.
A compound in which the valence bonds of the carbon atoms are not satisfied is said to be unsaturated. Such unsaturated molecules are important in the polymerization i.e., joining together of small molecules into large one having the same constituents.
7. What is polymeirsation?
Polymerisation is the process of forming a polymer.
8. Define the term 'degree of polymerisation'?
Degree of polymerization is the number of repetitive units (or mers) present in one molecule of a polymer.
Mathematically,
Degree of polymerization = Molecular weight of a polymer / Molecular weight of a single monomer
9. What is the difference between addition polymerization and condensation polymerization?
✔ Addition polymerization, also known as chain reaction polymerization, is a process by which two or more chemically similar monomers are polymerized to form long chain molecules.
✔ Condensation polymerization, also known as step-growth polymerization, is the formation of polymers by stepwise intermolecular chemical reactions that normally involve at least two different monomers.
10. Why are additives added to polymers?
The various polymer additives include:
1. Filler materials,
2. Plasticizers,
3. Stabilizers,
4. Colorants,
5. Flame retardants,
6. Reinforcements, and
7. Lubricants.
11. Why are the fillers and plasticizers added to polymers?

12. What are the characteristics of plastics which account for their wide use as engineering materials?
Plastics are extensively used in engineering applications due to their important properties such as low price, colour range, toughness, water resistance, low electrical and thermal conductivity, ease of fabrication, etc.
13. Differentiate commodity plastics with engineering plastics.
The plastics which are not generally used for engineering applications are known as commodity plastics. The plastics which are used in engineering applications are known as engineering plastics.
14. Name any four commodity plastics and engineering plastics.
Commodity plastics: (i) Polyethylene (PE), (ii) Polypropylene (PP), (iii) Polystyrene (PS), (iv) Polyvinyl chloride (PVC).
Engineering Plastics: (i) Ethenic, (ii) Polyamides, (iii) Cellulosics, (iv) Acetals.
15. Distinguish between thermoplastics and thermosetting plastics.
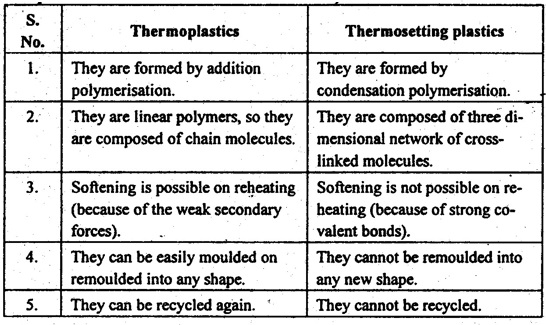
16. Name any four thermoplastics and thermosetting plastics.
Thermoplastics: Polythenes, polypropylene, polystyrenes, PVC.
Thermosetting plastics: Polyesters, phenolics, epoxides, melamine formaldehyde.
17. What advantages do thermoplastic polymers have over thermosetting polymers, and vice versa?
✔ Since thermoplastics have low melting temperature and can be repeatedly moulded and remoulded to the desired shape, they have a good resale/scrap value.
✔ The thermosetting plastics are generally stronger, harder, more brittle, more resistant to heat and solvents than thermoplastics.
18. What are the sources of raw materials for plastics?
1. Animal and vegetable by-products.
2. Coal by-products.
3. Petroleum by-products.
19. What do the following ‘acronyms' refer: PE, PP, PS, PVC, PTFE, PMMA.
PE: Polyethylene; PP: Polypropylene; PS: Polystyrene; PVC: Polyvinyl chloride; PTFE: Polytetrafluoro ethylene; PMMA: Polymethyl methacrylate.
20. List the properties and typical applications of PVC.
✓ Properties: Good low-cost, general purpose materials; ordinarily rigid, but can be made flexible with plasticizers; susceptible to heat distortion.
✓ Typical applications: Pipes, valves, fittings, floor tiles, wire insulations, toys, phonograph records, safety glass interlayers.
21. What are acrylic materials? Name two of them
✓ Acrylic materials are thermoplastic polymers based on the polymerization of esters of acrylic acid and/or methacrylic acid.
✓ The most commonly used acrylic polymers are:
1. PMMA (polymethyl methacrylate), and
2. PAN (polyacrylonitrile).
22. Write short notes on nylons.
✓ Polyamides (PA), also known as nylons, are the products of condensation reactions between an amine and an organic acid.
✓ There are number of common polyamides. They are usually designated as nylon 6, nylon 6/6, nylon 6/10, nylon 6/12, nylon 11, and nylon 12. These suffixes refer to the number of carbon atoms in each of the reacting substances involved in the condensation polymerization process.
23. What are bakelites? Also state their applications.
✓ Phenolics, also known as Bakelites, are the oldest family of thermosetting plastics. The most important phenolic materials is the polyformaldehydes.
✓ Typical applications include electrical plugs, sockets, switches, telephones, door knobs and handles, adhesives, coatings, and laminates.
24. List the characteristics of urea-formaldehyde.
1. They are similar to the phenolics.
2. They are hard and rigid thermosets.
3. They have good electrical insulation properties.
4. They exhibit good resistance to most chemicals.
5. They are light in colour.
25. What are engineering ceramics?
Engineering ceramics, are also known as technical/industrial ceramics, are those ceramics that are specially used for engineering applications or in industries.
26. List some of the distinct characteristics of engineering ceramics.
1. High resistance to abrasion and wear.
2. High strength at high temperature.
3. Good chemical stability.
4. Good electrical insulation characteristics.
27. What are the main classifications of ceramic materials?
Engineering ceramics can be classified into oxides, carbides, sulphides, nitrides, metalloids, or intermetallics.
28. Name any four engineering ceramics.
1. Alumina (Al2O3),
2. Silicon carbide (SiC),
3. Silicon nitride (Si3N4),
4. Partially stabilized zirconia (PSZ), and
5. Sialons.
29. Compare the fracture toughness of alumina, silicon carbide, and silicon nitride.
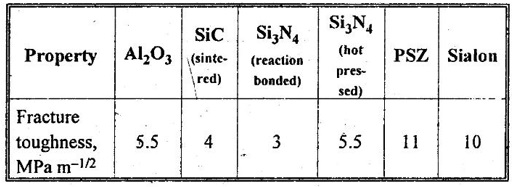
30. What is meant by PSZ?
Partially stabilized zirconia (PSZ) is nothing but a zirconium oxide (ZrO2), that has been blended and sintered with some other oxide such as magnesium oxide (MgO), calcium oxide (CaO), and yttria (Y2O3), to control crystal structure transformations.
31. What are sialons? State their applications.
✓ The name sialon is an acronym derived from the ingredients involved, namely Si Al-O-N. That is, the sialons are derivatives of silicon nitride. Sialons are formed when aluminium and oxygen partially substitute for silicon and nitrogen in silicon nitride.
✓ Sialons are used for cutting tool materials, dies for drawing wire and tubes, rock-cutting and coal-cutting equipment, nozzles and welding shields.
32. What are composites?
Composites are produced when two or more materials are joined to give a combination of properties that cannot be attained in the original materials.
33. What are the constituents of composites?
✓ Composites are composed of two phases. They are: 1. Matrix phase, and 2. Dispersed phase.
34. How are composite materials classified?
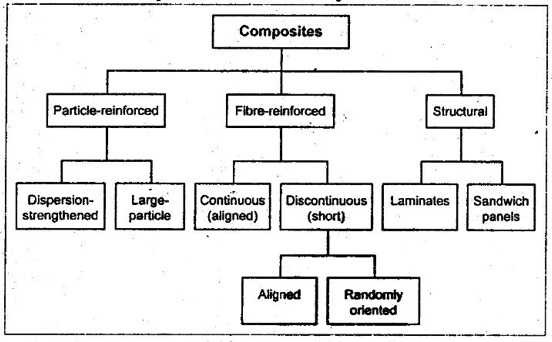
35. What is the role of matrix material in a composite?
The matrix usually provides the major control over electrical properties, chemical behaviour, and elevated-temperature use of the composite.
36. List the various matrix materials used.
1. Thermosetting resins: Polyester resins, epoxide resins
2. Thermoplastics: PA, PAI, PBT, PET, PES, PPS, PEEK
3. Metal matrices: Al, Ti, Mg, Cr and Ni, together with their alloys
4. Composite matrices
37. List the various fibre materials used in the fibre-reinforced composites.
1. Polymers: Kevlar, nylon, polyethylene
2. Metals: Be, Boron, W
3. Glass: E-glass, S-glass
4. Carbon: HS (high strength), HM (high modulus)
5. Ceramics: Al2O3, B4C, SiC, ZrO2
6. Whiskers: Al2O3, Cr, graphite, SiC, Si3N4
38. What are cermets? What are two common uses of cermets?
✓ The term 'cermet' refers to ceramic-metal composite containing between 80 and 90% of ceramic. Cermets are composed of ceramic particles in a metallic matrix.
✓ Typical applications: Cutting tools, slip gauge, wire- drawing dies, rocket motor and jet-engine parts.
No comments:
Post a Comment