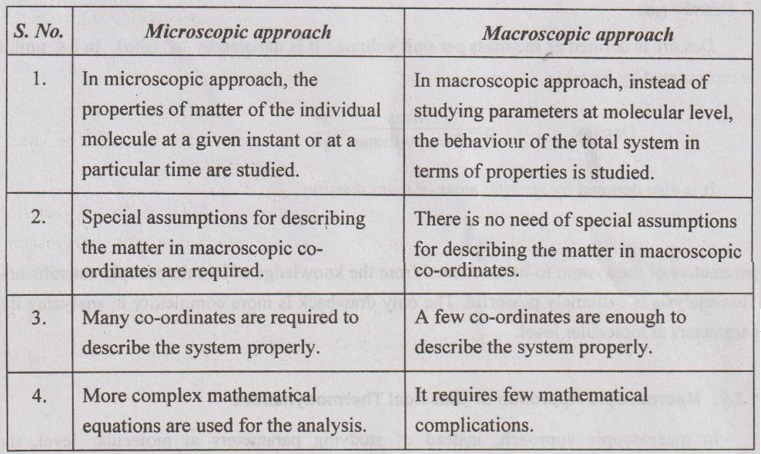
Thermodynamically, the behaviour of any matter can be studied in two different points of view: 1. Microscopic approach or statistical thermodynamics 2. Macroscopic approach or classical thermodynamics
MICROSCOPIC AND MACROSCOPIC APPROACHES
Thermodynamically, the behaviour of any matter can be studied in two different points of view:
1. Microscopic approach or statistical thermodynamics
2. Macroscopic approach or classical thermodynamics
1. Microscopic Approach or Statistical Thermodynamics
Any substance or matter consists of a large number of particles called molecules. The properties of substance naturally depend on the behaviour of these molecules.
From microscopic point of view, a matter consists of myriads of molecules. In this approach, the properties of matter such as pressure, velocity, temperature, position and energy of the individual molecule at a given instant or at a particular time are studied. This approach is known as statistical thermodynamics. For applications involving laser, plasma, high-speed gas flow, chemical kinetics, very low temperature (cryogenic) and others elements, the methods of statistical thermodynamics are essential.
For example, to investigate the statistical machinery, all results of classical thermodynamics are essential for a large number of results which enable the macroscopic parameters of the system to be calculated from the knowledge of its microscopic constituents. This analysis is extremely powerful. The only drawback is more complexity in analyzing the parameters at molecular level.
2. Macroscopic Approach or Classical Thermodynamics
In macroscopic approach, instead of studying parameters at molecular level, the behaviour of the total system in terms of properties such as pressure, volume, temperature, etc. are studied. These properties at every instant can easily be measured. It is also called macroscopic approach or classical approach. It is also known as classical thermodynamics.
For example, consider a system containing the mixture of air and petrol vapour in a cylinder of I.C. engine. The properties such as pressure, temperature and volumes are measured for each position of the piston in the cylinder. The state or conditions of the system i.e., mixture of air and petrol vapour can be determined by means of above-mentioned properties. These properties are called macroscopic properties. So, these properties describe the system condition in a macroscopic point of view.
The different coordinates are used to describe the various systems in a macroscopic approach. But, all coordinates should have the following characteristics:
1. There is no need of special assumptions for describing the matter in macroscopic co-ordinates.
2. Properties should be easily measurable quantities.
3. A few co-ordinates are enough to describe the system properly.
The classical approach is followed in forthcoming topics.
3. Comparison of Microscopic and Macroscopic Approaches
THERMODYNAMIC PROPERTIES
Thermodynamic properties describe the measurable characteristics of a substance. The knowledge of these properties is essential to the understanding of thermodynamics. The following are the thermodynamic properties.
1. Mass (m) and Weight (W):
Mass (m) is the measure of the amount of material present in a body. In S.I. unit, it is represented by kg.
Weight (W) of a body is the force exerted by the body when its mass is accelerated in a gravitational field. In S.I. unit, it is represented by N.
Weight, W= m × g
where m is the mass of the body and
g is the acceleration due to gravity.
The mass of a body remains the same regardless of its location in the universe. However, its weight changes with a change in gravitational acceleration. For example, on earth, an object has a certain mass and weight. When the same object is placed in outer space away from the earth's gravitational field, its mass is less or negligible.
2. Density (ρ):
Density is defined as the mass per unit volume. It is denoted by 'ρ' (rho). In S.I. unit, it is represented by kg/m3.
Density, ρ = Mass / Total volume = m / V
It is also denoted by specific mass or mass density.
3. Specific volume (v):
Specific volume is defined as the volume occupied by unit mass of the substances. It is the reciprocal of density. In S.I. unit, it is expressed as m3/kg.
Specific volume, v = Total volume / Mass = V / m
4. Specific weight (w):
Specific weight is defined as the weight per unit volume. In S.I. unit, it is expressed as N/m3 or kN/m3.
Specific weight, w = Total weight / Total volume = W / V
5. Specific gravity (s):
Specific gravity is defined as the ratio of density of a substance to the density of some standard substance at a specified temperature. Otherwise, it is the ratio of specific weight of the given substance to the specific weight of the standard substance at a specified temperature.
Specific gravity, s = Density (or) Specific weight of the given substance / Density (or) Specific weight of the standard substance
Normally, the standard substance for liquid is water and air is for gas. It has no unit because of the ratio of same units.
6. Pressure (p):
Pressure is defined as the force exerted per unit area. The pressure is considered as a property when a gas or a liquid is acalt. In the case of solids, the pressure is called stress. The fluid pressure increases with the depth because of the weight of fluid. But, the pressure in a tank containing gas may be considered as uniform since the weight of the gas is too small.
Pressure, p = Force / Area = F / A
Various units of pressure are as follows:
1 Pascal = 1 N/m2
1 bar = 105 N/m2 = 100 kN/m2
1 Torr = 1mm of Mercury (Hg)= 133.3 N/m2
1 mm of water (H2O) = 9.80665 N/m2
Atmospheric pressure (patm):
Atmospheric pressure is the pressure exerted by air on the atmosphere. Its value is taken at mean sea level as 1.01325 bar.
Atmospheric pressure = 1.01325 bar
= 101.325 kN/m2 or kPa
= 101325 N/m2or Pa
= 760 mm of Hg
= 10.34 m of water = 14.696 Psi
1 atm of pressure refers to 1.03125 bar or 101.325 kPa or 101325 Pa.
Gauge pressure (pg):
Gauge pressure is the pressure recorded by the pressure gauges. All pressure gauges read 'zero' pressure at atmospheric level. Hence, they actually measure the difference of fluid pressure and atmospheric pressure. The gauge pressures will be positive if it is above the atmospheric pressure and it will be negative if it is below atmospheric pressure.
Vacuum pressure (Pvac):
The pressure below atmospheric pressure is called vacuum pressure. It is also called negative pressure. A perfect vacuum would correspond to absolute zero pressure. The pressure gauge which is used to measure the vacuum pressure is called vacuum gauge.
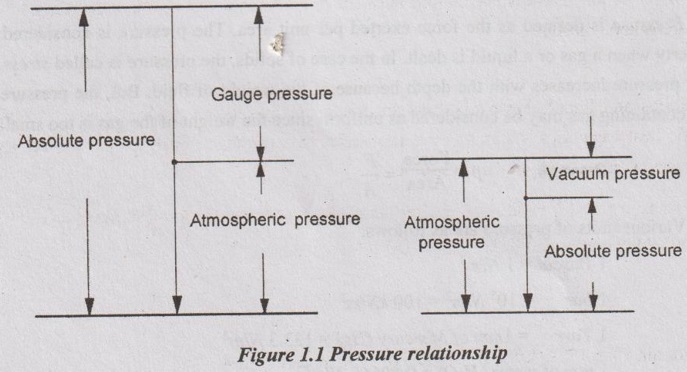
Absolute pressure (Pabs):
The pressure measured from absolute zero pressure is called absolute pressure.
Absolute pressure = Atmospheric pressure + Gauge pressure
Pabs = Patm+Pg
Absolute pressure = Atmospheric pressure - Vacuum pressure
Pabs = Patm - Pvac
1.7
Figure 1.1 shows the relationship between absolute, gauge, vacuum and atmospheric pressures.
8. Specific heat capacity (C):
Specific heat capacity is defined as "the quantity of heat required for raising or lowering the temperature of unit mass of the substance through one degree". It is denoted by C and expressed in J/kg K or kJ/kg K.
There are two specific heat capacities as follows:
(a) Specific heat capacity at constant volume (Cv):
Specific heat capacity at constant volume is defined as "the quantity of heat required for raising or lowering the temperature of unit mass of the substance through one degree when the volume remains constant." It is denoted by Cv.
(b) Specific heat capacity of constant pressure (Cp):
Specific heat capacity of constant pressure is defined as "the quantity of heat required to raise or lower the temperature of unit mass of the substance through one degree when the pressure is kept constant."
Note:
Solids and liquids have only one specific heat capacity. Gases and vapours have two specific heat capacities. Cp and Cv are properties of a substance but they are not constants. They vary with pressure and temperature.
For any gas, Cp is always greater than Cv. The ratio of two specific heats remains constant and denoted by the symbol 'γ' (gamma).
γ = Cp / Cv
For air, Cp = 1.005 kJ/kg K, Cv = 0.718 kJ/kg K and γ = 1.4.
No comments:
Post a Comment