Heat is defined as the energy crossing the boundary of a system due to the temperature difference between system and surroundings.
HEAT TRANSFER (Q)
Heat is defined as the energy crossing the boundary of a system due to the temperature difference between system and surroundings. It is usually represented by Q and expressed in Joule or kJ.
Heat exists only due to the heat transfer. For the transfer of heat, there should be temperature difference between two systems. The amount of heat transferred depends upon the path and not simply on the initial and final conditions of the system. Also, it is important to distinguish between heat added to a system from its surroundings and heat removed from a system to its surroundings. A positive value of heat indicates that the heat is added to the system by its surroundings called heat addition. A negative value of heat indicates the transfer of heat out of a system to its surroundings called heat rejection.
The best way to quantify the definition of heat is to consider the relationship between the amount of heat added to a system or removed from a system and the change in the temperature of the system. When a substance is heated, its temperature increases and when it is cooled, its temperature decreases. The heat added to a substance or removed from a substance to produce a change in its temperature is called sensible heat. The units of heat are defined in terms of the change in temperature as it produces.
Let m be the mass of the substance heated from temperature T1 to temperature T2, then, the heat transfer is given by
Q = m. C. (T2 - T1) = m C dT ... (1.4)
where C be the specific heat of the substance.
The unit of heat transfer is J (Joule) or kJ
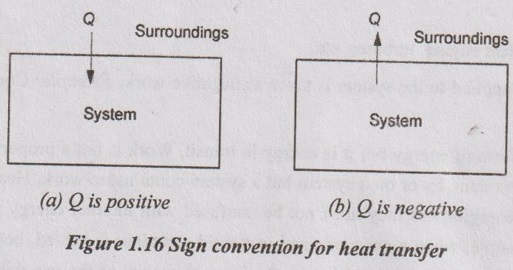
Sign conventions:
If Q is positive, the heat is supplied or transferred to a system.
If Q is negative, the heat is rejected or transferred from a system.
No comments:
Post a Comment