Property is defined as any measurable or observable characteristics of the substance when the system remains in equilibrium state.
INTENSIVE AND EXTENSIVE PROPERTIES OF A SYSTEM
Property is defined as any measurable or observable characteristics of the substance when the system remains in equilibrium state. Any characteristic of a system is called its property.
Example: Pressure (p), temperature (T), volume (V), mass (m), etc.
The properties are classified into the following two types:
(a) Intensive or intrinsic property and
(b) Extensive or extrinsic property.
(a) Intensive properties:
Intensive properties are independent of the mass of the system. If a part of the system is considered, these properties remain the same.
Example: Pressure, temperature, specific volume, density etc.
(b) Extensive properties:
Extensive properties are dependent upon the mass of the system. If a part of the system is considered, these properties have less value.
Example: Mass, volume, total energy, weight, kinetic energy, etc.
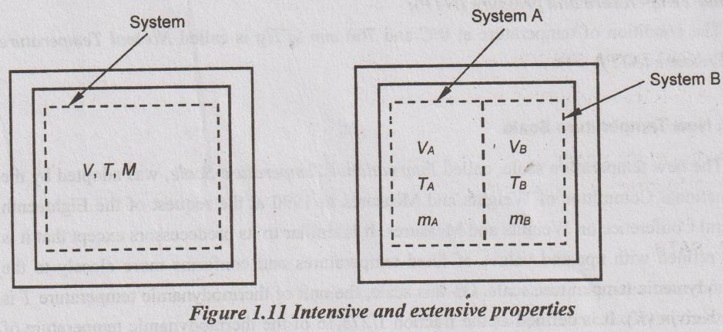
An easy way to determine whether a property is intensive or extensive is to divide the system into two equal parts with an imaginary partition as shown in Figure 1.11. Each part will have the same value of intensive properties as the original system but half of the value of the extensive properties.
V = VA + VB ⇒ Extensive property
m = mA + mB ⇒ Extensive property
T ≠ TA + TB ⇒ Intensive property
No comments:
Post a Comment