Iron has been widely used by man for thousands of years.
IRON-CARBON SYSTEM
1. Introduction
Iron has been widely used by man for thousands of years. The availability comparatively low cost, and the wide range of properties have made irons and steels materials of great importance. Steels↑ and cast irons↑↑ containing varying percentages of carbon and other
†The alloys of iron-carbon system containing 0.06% to 2.0% carbon are known as steels.
†† Those alloys having carbon from 2.0% to 6.7% are called cast-irons.
alloying elements are very popular engineering materials. In fact, irons and steels account for more than 90% of the total tonnage of all metals used today.
Because of its outstanding commercial importance, the study of iron-carbon alloys in detail is very essential for the engineers.
2. Allotropic Forms of Pure Iron
✓ Pure iron is a allotropic metal.
✓ Allotropy refers to the possibility of existence of two or more different crystal structures for a substance depending upon temperature. This phenomenon is also known as polymor- phism.
Constitution of Alloys and Phase Diagrams 1.47
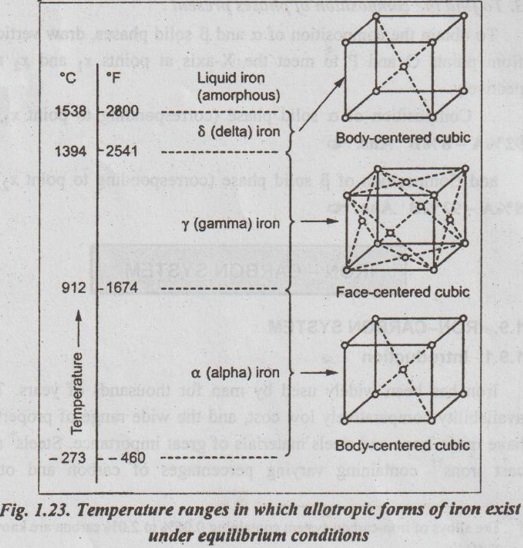
✓ The pure iron exists in three allotropic forms i.e., α iron, γ iron, and δ iron.
✓ Fig.1.23 depicts the temperature ranges in which allotropic foods forms of pure iron exist under equilibrium conditions.
✓ From the Fig.1.23, for pure iron the ranges are:
(i) α-iron - Body Centred Cubic (BCC) - Stable at temperatures up to 908°C
(ii) γ-iron - Face Centred Cubic (FCC) - Stable between 908°C and 1388°C.
(iii) δ-iron-Body Centred Cubic (FCC) - Stable between 1388°C and 1535°C (the melting point).
Note
The temperatures at which the structural changes take place are called critical points or arrest points.
No comments:
Post a Comment