When a gas undergoes a process from initial state to final state, the thermodynamic properties will change.
POINT FUNCTION AND PATH FUNCTION
When a gas undergoes a process from initial state to final state, the thermodynamic properties will change. Some of the properties such as pressure, volume and temperature are not dependent on the path followed by a system. It is purely independent of the path followed by a process. These properties are called point function or state function.
Example: Pressure, volume, temperature, etc.
In Figure 1.15, the properties such as pressure and volume do not depend upon the path [a-1-b (or) a-2-b] followed by the gas. It requires end points only. Therefore, these properties are called point function.
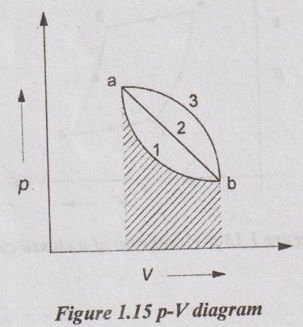
Some properties such as work transfer, heat transfer, etc. are dependent on the path followed by a gas. These properties are called path function. In Figure 1.15, for path a-1-b, the work transfer is less than the path a-3-b. Therefore, it is dependent of the path followed by a gas.
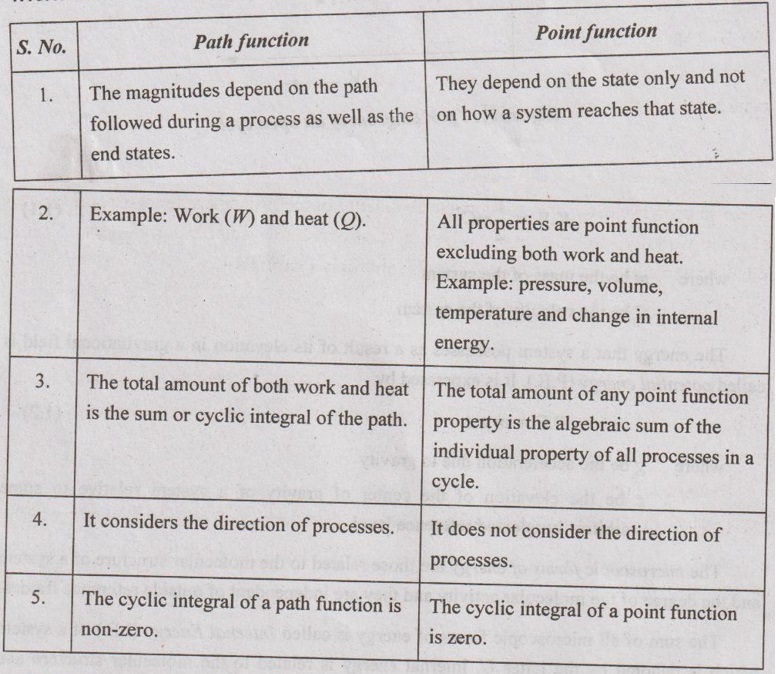
1. Difference between Path and Point Functions
No comments:
Post a Comment