A thermodynamic system is defined as a definite space or area on which the study of energy transfer and energy conversions is made.
THERMODYNAMIC SYSTEMS
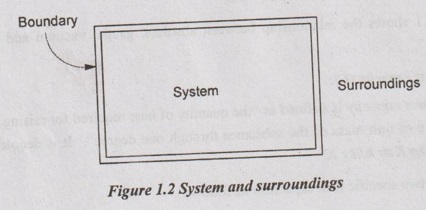
System
A thermodynamic system is defined as a definite space or area on which the study of energy transfer and energy conversions is made. A system could be the water within the turbine or the gas inside a length of pipe or the entire lubricating oil system for a petrol engine.
2. Surroundings
Anything outside the system which affects the behaviour of the system is known as surroundings or environment. For example, a fan may be the system but the surrounding is air in the house and entire universe.
3. Boundary
The system and surroundings are separated by a boundary. It may be fixed or movable one. Also, it may be real or imaginary one. At the same time, the boundary is the contact surface which is shared by both system and surroundings. It has zero thickness. It will not occupy any volume or mass in space,
Permeable process boundaries allow the mass transfer to occur. The mass transfer cannot occur across impermeable boundaries.
A diathermal boundary allows the heat transfer to occur across it which is similar to thin metal walls. The heat transfer cannot occur across the adiabatic boundary.
A moveable/deforming boundary is capable of performing "boundary work". No boundary work transfer can occur across a rigid boundary. However, the energy transfer can still occur via shaft work, e.g., through the stirring of fluid in a blender.
4. Universe
A system and surroundings together comprise a universe. System and surroundings when put together result in universe.
Universe = System + Surroundings
No comments:
Post a Comment