Process is the change of state undergone by a system from one equilibrium state to another equilibrium state.
THERMODYNAMIC PROCESS
Process is the change of state undergone by a system from one equilibrium state to another equilibrium state.
It consists of sufficient information about the thermodynamic properties at successive state points. The path of the succession of states through which the system passes is called thermodynamic process. For example, (i) increasing the temperature of a fluid while maintaining a constant pressure and (ii) increasing the pressure of a confined gas while maintaining a constant temperature.
The series of states through which a system passes during a process is called path of process. To describe a process completely, one should specify the initial and final states of the process as well as path it follows.
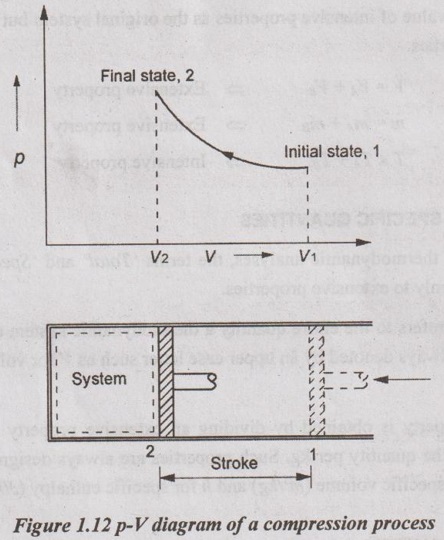
Process diagrams, plotted by employing thermodynamic properties as coordinates, are very useful in visualizing the processes. Some common properties which are used as coordinates are temperature T, pressure p and volume V (or specific volume v). Figure 1.12 shows a p-V diagram of the compression process of a gas.
1. Quasi-Static Process
In a quasi-static process, a system passes through an infinite number of continuous equilibrium states and attains the original state when the process is reversed.
A quasi-state process follows a successive thermodynamic equilibrium process called reversible process. It is very slow process.
2. Reversible Process
A reversible process is defined as a process in which a system passes through an infinite number of continuous equilibrium states and it follows the same path when this process is reversed. When a fluid undergoes reversible process, both fluid and its surroundings can always be restored to their original state. In reality, there are no truly reversible processes. However, one uses the reversible process to make the analysis simpler and to determine the maximum theoretical efficiencies for analysis purposes.
Example: Constant volume, constant pressure, isothermal, adiabatic process, isentropic process, etc.
3. Irreversible Process
An irreversible process is defined as a process in which a system passes through an infinite number of continuous non-equilibrium states and it does not follow the same path when the process is reversed. For example, an automobile engine does not give back the fuel taken to drive up a hill when it drives back down the hill.
Example: Free expansion, throttling and steady flow process.
There are many factors that make a process irreversible. Four of the most common causes of irreversibility are friction, unrestrained expansion of a fluid, heat transfer through a finite temperature difference and mixing of two different substances. These factors are present in real and irreversible processes. Also, these factors prevent these processes from being reversible.
4. Flow Process
In a flow process, the working fluid enters the system and leaves it to atmosphere after doing the work. In this system, both energy and mass cross the boundary.
Example: Steady flow process applied to various systems such as boilers, turbine, compressor etc.
5. Non-flow Process
In a non-flow process, the same working fluid is recirculated again and again within the system. It does not leave the system after doing the work. In this system, only the energy crosses the boundary in the form of heat and work.
Example: Constant volume process, constant pressure process, isentropic process, isothermal process, etc.
No comments:
Post a Comment