A process in which a fluid successively changes state for the purpose of producing or transferring energy is known as cycle.
THERMODYNAMIC CYCLE
A process in which a fluid successively changes state for the purpose of producing or transferring energy is known as cycle. A thermodynamic cycle consists of a linked sequence of thermodynamic processes and eventually returns the system to its initial state. The thermodynamic processes in a cyclic involve transfer of heat and work into and out of the system, while varying pressure, temperature, and other state variables within the system.
There are two types of cyclic processes.
(a) Closed cycle
(b) Open cycle.
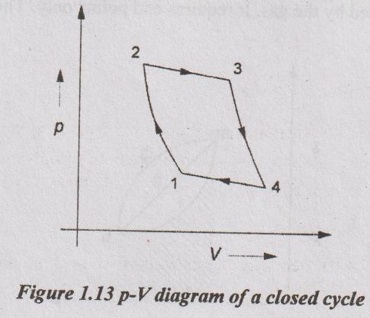
1. Closed Cycle
During a closed cycle, the system returns to its original thermodynamic state of temperature and pressure. In a closed cycle, the working substance is recirculated again and again within the system itself without taking any mass transfer. It is shown in Figure 1.13.
2. Open Cycle
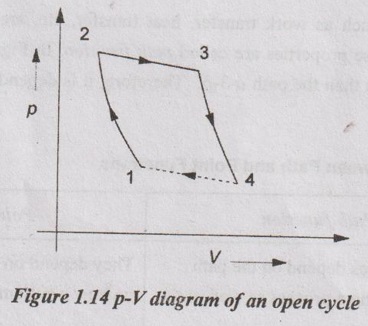
In an open cycle, new mass enters the boundaries of the system and spent exhaust leaves it i.e. the working substance is exhausted to atmosphere after completing the process. So, both mass and energy transfers take place. It is shown in Figure 1.14.
No comments:
Post a Comment