Temperature is the measure of molecular velocity of fluid particles.
CONCEPT OF TEMPERATURE AND THERMAL EQUILIBRIUM
1. Temperature
Temperature is the measure of molecular velocity of fluid particles. The greater is the movement of molecules, the higher will be the temperature. There is no exact definition for temperature. It is a property which is used to determine the degree of hotness or coldness or the level of heat intensity of a body. Based on physiological sensations only, the level of temperature is qualitatively expressed in words such as freezing cold, cold, warm, hot and red-hot. Several properties change with temperature such as viscosity, surface tension, density, etc., of materials change with temperature in a repeatable and predictable way and it forms the basis for accurate temperature measurement.
Temperature is commonly measured by using a mercury-in glass thermometer. It can also be measured by using several other temperature dependent properties.
2. Thermal Equilibrium
A system is said to be in thermal equilibrium when there is no temperature difference throughout the system. It is a common experience that a cup of hot tea left on the table eventually cools off and a cold drink eventually warms up. It means, when a body is brought into contact with another body which are at different temperatures, the heat is transferred from the body at higher temperature to the one at lower temperature until both bodies attain the same temperature. At that point, the heat transfer stops and two bodies attains thermal equilibrium. The equality of temperature is the only requirement for thermal equilibrium.
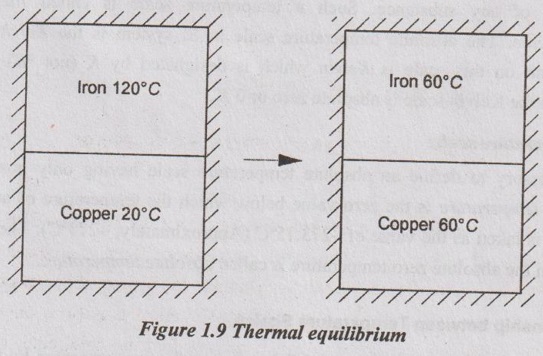
To illustrate this, consider an iron block at higher temperature of 120°C and another copper block at lower temperature of 20°C as shown in Figure 1.9. If the blocks are brought into contact and isolated from their surroundings, there would be the transfer of heat from high temperature iron block to low temperature copper block. During this process, the temperature of the warm-block decreases with time while the temperature of the cold-block increases with time. When both blocks reach the equal temperature, the heat transfer will end. So, the two blocks are then in thermal equilibrium.
3. Temperature Scales
Temperature scales provide a common basis for temperature measurements. All temperature scales are based on easily reproducible states such as freezing and boiling points of water which are also called ice point and steam point respectively.
At the ice point, a mixture of ice and water is in equilibrium with air saturated with vapor at 1 atm pressure.
At the steam point, a mixture of liquid water and water vapor (with no air) is in equilibrium at 1 atm pressure.
Fahrenheit (F) and Celsius (C) scales:
The two temperature scales normally employed for the measurement purpose are Fahrenheit (F) and Celsius (C) scales. These scales are based on a specification of the number of increments between ice point and steam point of water at standard atmospheric pressure. The Celsius scale has 100 units between these points and the Fahrenheit scale has 180 units. The zero points on the scales are arbitrary. On the Celsius scale, the ice and steam points are originally assigned the values of 0°C and 100°C respectively. The corresponding values on the Fahrenheit scale are 32°C and 212°F. These are referred as two-point scales since the temperature values are assigned at two different points.
Thermodynamic temperature scale:
In thermodynamics, it is necessary to have a temperature scale which is independent of the properties of any substance. Such a temperature scale is called thermodynamic. temperature scale. The absolute temperature scale in SI system is the Kelvin scale. The temperature unit on this scale is Kelvin which is designated by K (not °K). The lowest temperature on the Kelvin scale is absolute zero or 0 K.
Absolute temperature scale:
It is necessary to define an absolute temperature scale having only positive values. Absolute zero temperature is the zero value below which the temperature of any substance cannot fall. It is taken as the value of -273.15°C (Approximately, -273°C). The temperature measured from the absolute zero temperature is called absolute temperature.
4. Relationship between Temperature Scales
The relationship between Celsius and Fahrenheit scales is represented by the following equations:
°F 32.0+(9/5) °C
°C = (°F - 32.0) (5/9)
Celsius scale is converted into Kelvin scale as follows.
T = t + 273 K
where
T - Temperature on Kelvin scale
t - Temperature on Celsius scale.
Figure 1.10 shows the relationships between Celsius (°C) scale and Kelvin (K) scale.
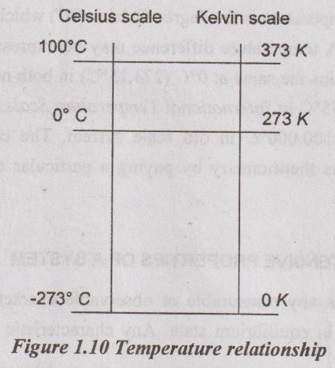
The magnitudes of each division of 1 K and 1°C are identical. Therefore, the temperature interval on both scales is the same when the temperature differences ΔT are considered. Raising the temperature of a substance by 10°C is the same as raising it by 10 K. It means,
ΔT (K) = ΔT (°C)
Standard Temperature and Pressure (STP):
The standard atmospheric conditions are:
Standard temperature = 15°C
Standard pressure = 760 mm of Hg = 101.325 kN/m2
Normal Temperature and pressure (NTP):
The condition of temperature at 0°C and 760 mm of Hg is called Normal Temperature and Pressure (NTP).
5. New Temperature Scale
The new temperature scale, called International Temperature Scale, was adopted by the International Committee of Weights and Measures in 1990 at the request of the Eighteenth General Conference on Weights and Measures. It is similar to its predecessors except that it is more refined with updated values of fixed temperatures and conforms more closely to the thermodynamic temperature scale. On this scale, the unit of thermodynamic temperature T is again Kelvin (K). It is defined as the fraction 1/273.16 of the thermodynamic temperature of the triple point of water.
The unit of Celsius temperature is the degree Celsius (°C) which is by definition equal in magnitude to Kelvin (K). A temperature difference may be expressed in Kelvin or degrees Celsius. The ice point remains the same at 0°C (273.15°C) in both new and old scale systems but the steam point is 99.975°C in International Temperature Scale (with an uncertainly of ± 0.005°C) whereas it was 100.000°C in old scale system. The change is due to precise measurements made by gas thermometry by paying a particular attention to the effect of sorption.
No comments:
Post a Comment