Hardening refers to the heat treatment of steel which increases its hardness by quenching.
HARDENING (BY QUENCHING)
1. What does Hardening refer?
✓ Hardening refers to the heat treatment of steel which increases its hardness by quenching.
✓ Hardening normally implies heat-treating operations which produce microstructures which are entirely or predominantly martensitic.
2. Objects of Hardening
To The main purposes of hardening are:
1. To harden the steel to resist wear.
2. To enable it to cut other metals.
3. Operation
The process of hardening involves the following stages:
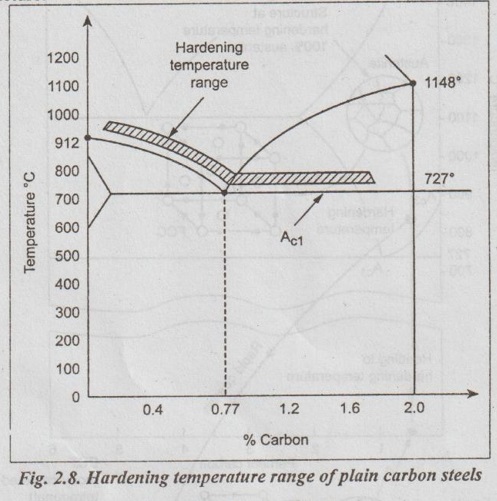
1. Heating: The steel to be heat treated is heated slowly in a furnace to a temperature 30°C to 50°C above the upper critical temperature i.e., above the A3 line, as shown in Fig.2.8.
2. Soaking: The heated steel is held at this temperature for considerable length of time to allow complete austenisation.
3. Cooling: The steel is cooled by quenching (in a water bath or oil bath) to the room temperature. The cooling rate should be higher than the critical cooling rate in order to get the completely martensitic structure.
4. Phase Transformation During Hardening
✓ Due to rapid cooling, the austenite (γ iron) will be super cooled by nearly 500°C and the driving force will be large enough to convert FCC body centered tetragonal (BCT), as shown in Fig.2.9. The resulting structure of FCC BCT is called as martensite.
✓ A new phase transformation from austenite to martensite occurs without a compositional change.
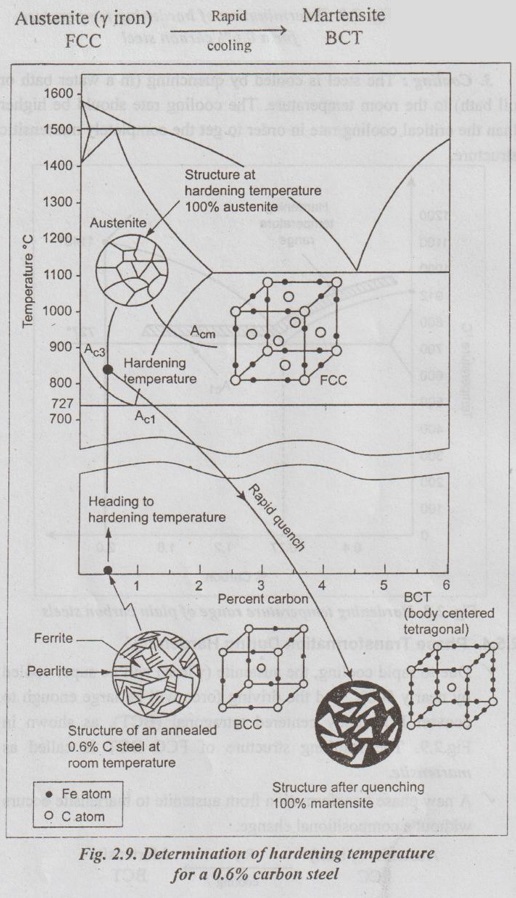
✓ The martensite is super-saturated solution of carbon in a-iron. Also because carbon is present in the lattice, slip does not occur easily. That's why, martensite is very hard, strong and brittle. It has needle like structure.
5. Factors Affecting the Hardness
The hardness obtained from the hardening process depends upon the following factors:
1. Carbon content,
2. Quenching medium,
3. Specimen size, and
4. Other factors.
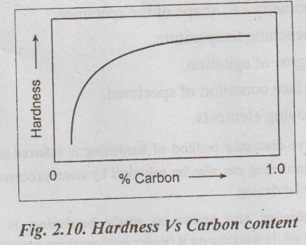
1. Carbon Content
✓ Since the hardness in steel is due to carbon content, therefore usually the hardening process is carried out on the high carbon steels (0.35 to 0.50%C).
✓ As the carbon content in steel increases, the hardness obtainable also increases, as shown in Fig.2.10.
2. Quenching Medium
✓ As we know, the hardness depends essentially upon the nature and properties of quenching medium.
✓ It may be noted that faster the cooling the greater the hardness, Too slower the cooling lower the hardness.
✓ As we discussed in Section 2.4, the quenching in water or brine solution is commonly used for hardening low and medium plain carbon steels. For high carbon and alloy steels, mineral oil is generally used as the quenching medium, because its action is not so severe as that of water. Certain alloy steels can be hardened by air cooling.
3. Specimen Size
✓ The specimen size also affects the hardness obtained from hardening process.
✓ As the specimen size increases, the hardness decreases even though all other conditions have remained the same. For example, a 50 mm diameter steel bar will attain greater ole hardness than a 100 mm diameter steel for of similar nodi composition.
4. Other Factors
The other factors that affect the hardness obtained from hardening process include:
(i). Geometry i.e., shape of the specimen.
(ii) Quenching temperature.
(iii) Degree of agitation.
(iv) Surface condition of specimen.
(v) Alloying elements.
Note
The above discussed method of hardening is referred as hardening by quenching. The hardening can also be achieved by other processes such as work hardening and age hardening.
1. Work hardening, also known as strain hardening, is the process of hardening a metal, while working on it (under cold-working conditions).
2. Age hardening, also known as precipitation hardening, is the process of hardening a metal when allowed to remain or age after heat treatment. For more details, refer Unit 3, Section 3.27.
No comments:
Post a Comment