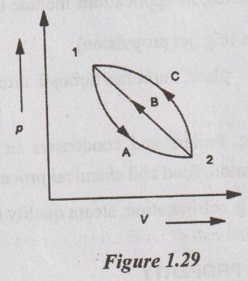
Consider a system changing from state 1 to state 2 by following the path A but the system returns to the original state in two different paths namely B and C as shown in Figure 1.29.
INTERNAL ENERGY IS A PROPERTY
Consider a system changing from state 1 to state 2 by following the path A but the system returns to the original state in two different paths namely B and C as shown in Figure 1.29.
As per the first law of thermodynamics, for path A
QA = WA + ΔUA
For path B, QB = WB + ΔUB
For path C, QC = WC + ΔUC
For the cycle 1-A-2-B-1, we know that

Similarly, for the cycle 1-A-2-C-1,

From equations (1.33) and (1.34), it can be written as
ΔUB = ΔUC
It indicates that the change in internal energy is constant between two states irrespective of the path followed by the system. Hence, the internal energy is a property of the system.
Sign conversions:
(a) If ΔU increases, it is taken as positive.
(b) If ΔU decreases, it is taken as negative.
Limitations of first law of thermodynamics:
(a) First law of thermodynamics does not specify the direction of flow of heat and work. i.e., whether the heat flows from a hot body to a cold body or from a cold body to a hot body.
(b) The heat and work are mutually convertible. The work can be converted fully into heat energy but the heat energy cannot be converted fully into mechanical work. It violates the foresaid statements. A machine which violates the first law of thermodynamics is known as Perpetual Motion Machine (PMM-1) of the first kind which is impossible.
PMM-1 is a machine which delivers work continuously without any input. Thus, the machine violates first law of thermodynamics.
No comments:
Post a Comment