Quenching refers accelerated cooling.
QUENCHING
1. What is Quenching?
✓ Quenching refers accelerated cooling.
✓ The cooling can be accomplished by contact with a quenching medium which may be a gas, liquid, or solid.
✓ Most of the times, liquid quenching media is widely used to achieve rapid cooling.
2. Types of Quenching Medium
Some of the quenching medium that are used generally in industries, in order of decreasing quenching severity↑, are given below:
1. 5-10% caustic soda
2. 5-20% brine (NaCl)
3. Cold water
4. Warm water
5. Mineral oil (obtained during the refining of crude petroleum)
6. Animal oil
7. Vegetable oil (such as linseed, cottonseed, and rapeseed)
8. Air
Thus water produces the most severe quench followed by oil, which is more effective than air.
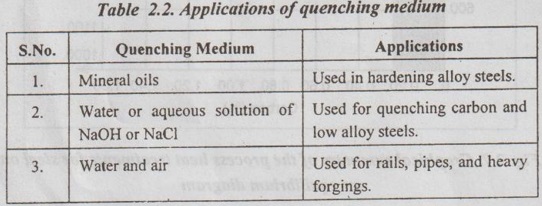
Table 2.2 shows the applications of some of the quenching medium.
Table 2.2. Applications of quenching medium
† 'Severity of quench' is a term used to indicate the rate of cooling; the more rapid the quench, the more severe the quench.
3. Selection of Quenching Medium
The selection of quenching medium is based on the following factors:
1. Desired rate of heat removal.
2. Required temperature interval.
3. Boiling point.
4. Viscosity.
5. Flash point (if combustible).
6. Stability under repeated use.
7. Possible reactions with the material being quenched.
8. Cost.
4. Stages of Quenching
The three stages of quenching are:
Stage 1: Vapour-Jacket Stage
✓ When a piece of hot metal is first inserted into a tank of liquid Hoirly quenchant↑, that adjacent to the metal vapourises and forms a gaseous layer separating the metal and liquid.
✓ In this stage cooling is slow since all heat transport must be through a gas (by conduction and radiation).
✓ This stage occurs when the metal is above the boiling point of the quenchant.
Stage 2: Vapour-Transport Cooling Stage
✓ This stage starts when the hot metal is cooled to a temperature at which the gaseous layer is no longer stable.
✓ In this stage, bubbles nucleate and remove the gaseous layer; liquid contacts the metal and vapourises it and thus the process of bubbles formation continues.
† Quenchant means quenching medium.
✓ This second stage of quenching provides rapid cooling as a result of the large quantities of heat removed by the mechanism.
Stage 3: Liquid Cooling Stage
✓ Third stage begins when the metal cools below the boiling point of the quenchant.
✓ In this stage, all heat transfer occurs through conduction across the solid-liquid interface, aided by convection or stirring within the quenchant.
✓ The third stage of quenching provides the slowest rate of cooling.
No comments:
Post a Comment