There are different microscope constituents of iron-carbon alloys exist.
MICRO-CONSTITUENTS OF IRON-CARBON ALLOYS
✓ There are different microscope constituents of iron-carbon alloys exist. The study of these micro-constituents is essential in order to understand iron-iron carbide (Fe-Fe3C) equilibrium phase diagram.
✓ Various micro-constituents of iron-carbon alloys are:
1. Ferrite,
2. Austenite,
3. Cementite,
4. Pearlite,
5. Ledeburite,
6. Martensite,
7. Troostite,
8. Sorbite, and
9. Bainite.
Now we shall present a brief note on the above constituents in the following sections.
1. Ferrite (or α-iron)
✓ Ferrite is a primary solid solution based on a iron having BCC structure.
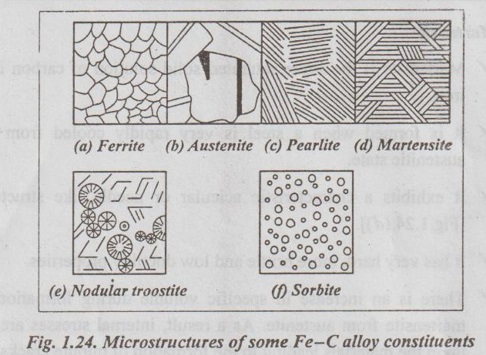
✓ It is nothing but the interstitial solid solution of carbon in iron. [Fig.1.24 (a)].
✓ Maximum solubility of carbon in iron is 0.025% carbon at 723°C, while its solubility at room temperature is only about 0.008%.
✓ Ferrite is soft, ductile, and highly magnetic.
✓ It can undergo extensive cold working.
2. Austenite (or γ-iron)
✓ Austenite is a primary solid solution based on γ iron having FCC structure.
✓ This is also an interstitial solid solution of carbon in iron. [Fig.1.24 (b)]
✓ The maximum solubility of carbon in FCC iron is about 2% at 1140°C.
✓ Austenite is normally not stable at room temperature; mostly stable only above the critical temperatures.
✓ Austenite is soft, tough, and highly ductile. Thus it is more suitable for fabrication processes.
✓ It is also a non-magnetic (paramagnetic).
✓ Austenite has a greater electrical resistance and coefficient of expansion than ferrite.
3. Cementite
✓ Cementite is the name given to the carbide of iron (Fe3C).
✓ It is the hard, brittle, intermetallic compound of iron with 6.69% of carbon.
✓ The hardness and brittleness of cast iron is believed to be due to the presence of the cementite.
✓ It is magnetic below 250°C.
(a) Ferrite
(b) Austenite (c) Pearlite (d) Martensite
(e) Nodular troostite
4. Pearlite
✓ Pearlite is the eutectoid mixture of ferrite (87.5%) and cementite (12.5%).
✓ It is formed when austenite decomposes during cooling. It contains 0.8% of carbon.
✓ It consists of alternate thin layers (or lamellae) of ferrite and cementite, as shown in Fig. 1.24 (c).
✓ The name derives from its lustrous appearance (similar to mother-of-pearl) when viewed in white light under a microscope.
✓ The properties of pearlite is midway between ferrite and cementite. It is relatively strong, hard and ductile.
5. Ledeburite
✓ Ledeburite is the eutectic mixture of austenite (γ iron) and cementite (Fe3C) containing 4.3% carbon.
✓ In pure iron-carbon alloy, it forms at 1140°C. Most of engineering alloy materials belong to this range of alloy.
✓ Pig iron, the most important engineering material, is ledeburite.
6. Martensite
✓ Martensite is the super saturated solid solution of carbon in a iron.
✓ It is formed when a steel is very rapidly cooled from the austenitic state.
✓ It exhibits a characteristic acicular or needle like structure. [Fig.1.24 (d)].
✓ It has very hard, more brittle and low ductility properties.
✓ There is an increase in specific volume during formation of martensite from austenite. As a result, internal stresses are set up in the materials leading to the formation of minute cracks.
7. Troostite
✓ Troostite is the mixture of radial lamellae of ferrite and cementite. In fact, it differs from pearlite only in the degree of fineness.
✓This constituent is also known as troostite pearlite.
✓ It is the microstructure consisting ferrite and finely divided cementite, produced on tempering martensite below 450°C [Fig.1.24 (e)].
✓ It is formed by the decomposition of austenite when cooled at a rate slower than that which will yield a martensitic structure and faster than that which will produce a sorbitic structure.
✓ It has hardness intermediate between martensite and sorbite.
8. Sorbite
✓ Sorbite is the microstructure consisting ferrite, and finely divided cementite, produced on tempering martensite above 450°C.
✓ This constituent is also known as sorbitic pearlite.
✓ It is formed by the decomposition of austenite when cooled at a rate slower than that which will yield a troostitic structure and faster than that which will produce a pearlitic structure [Fig.1.24 (f)].
✓ Though sorbitic steel is, slightly less ductile than pearlite steel, its tensile and yield strengths are high. Thus sorbitic steels are often known as 'toughened steels'.
Note
All the pearlite, troostite and sorbite are ferrite-cementite mixtures having a lamellar structure. However, they are distinguished by their degree of dispersion. Pearlite has coarse pearlite; troostite has fine pearlite; and sorbite has medium pearlite.
9. Bainite
✓ Bainite is a decomposition product of autenite, consisting of an aggregate of ferrite and carbide.
✓ Bainite obtained by transformation of pearlite at higher temperature (has a feathery structure) is called upper bainite.
✓ Bainite obtained by low temperature transformation (has needle like or a acicular structure) is called lower bainite.
✓ Lower bainite provides high mechanical properties and that is why it is extensively used for components of machine and structures.
✓ Bainite has hardness in between the hardness of pearlite and martensite.
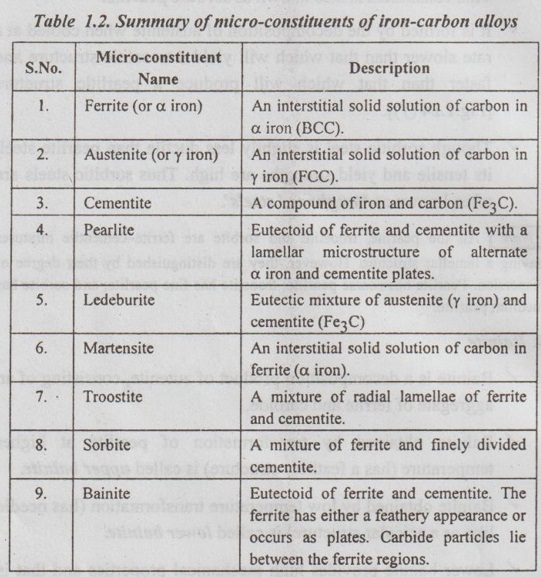
Summary of Micro-Constituents of Iron-Carbon Alloys
Table 1.2 summarises the various microstructures that commonly occur when Fe-C alloys are heat treated.
No comments:
Post a Comment