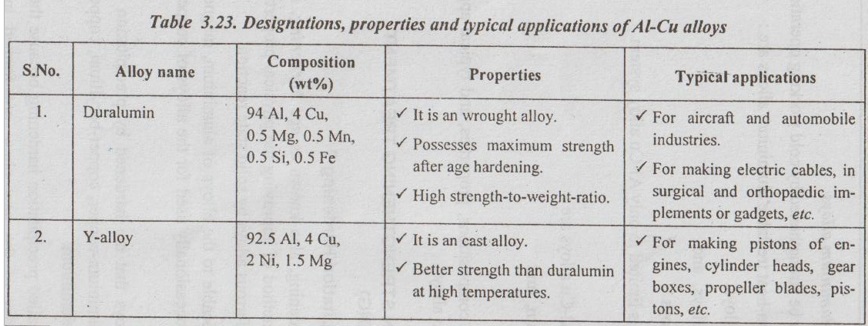
Precipitation hardening, also known as age hardening, is the most important method of improving the physical properties of some of the non-ferrous alloys by solid state reaction.
PRECIPITATION STRENGTHENING TREATMENT
(AGE HARDENING)
1. What is Precipitation Hardening?
✓ Precipitation hardening, also known as age hardening, is the most important method of improving the physical properties of some of the non-ferrous alloys by solid state reaction.
✓ It is mostly applicable to the alloys of aluminium, magnesium and nickel. It is occasionally used for the alloys of copper and iron.
✓ Examples of alloys that are hardened by precipitation treat- ments include aluminium-copper, copper-beryllium, copper-tin, and magnesium-aluminium.
✓ This process is called precipitation hardening because the fine precipitate particles of the new phase are formed in this hardening process.
Note
Another Al-Cu alloy is aldural, also known as alclad. It is a duralumin with a thin coating of pure aluminium. The thickness of layer of pure aluminium is about 5% of thickness of core and such a layer prevents corrosion due to salt water.
2. Process of Precipitation Heat Treatment
The process of precipitation heat treatment consists of three steps. The three step process is explained for an aluminium alloy, say Al-4% Cu alloy (called duralumin) below.
Fig.3.9 shows the Al-Cu phase equilibrium diagram. Fig.3.9 also shows the three steps in the precipitation hardening heat treatment together with the microstructures that are produced.
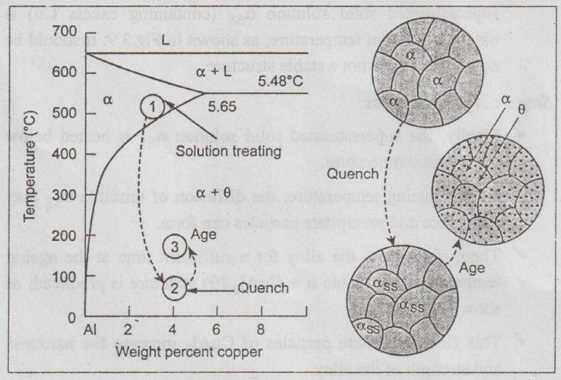
Fig. 3.9. The aluminium-rich end of the aluminium-copper phase diagram showing the three steps in the age-hardening heat treatment and the microstructures that are produced
Step 1: Solution treatment
✓ First the alloy is heated above the solvus temperature to obtain its solid solution.
✓ The alloy is held at this temperature until a homogeneous solid solution α is produced.
✓ This step dissolves the θ precipitate and reduces any segregation present in the original alloy.
✓ As shown in Fig.3.9, the Al - 4% Cu alloy is solution-treated between 500°C and 548°C.
Step 2: Quenching process
✓ After solution treatment, the alloy (which contains only α structures) is quenched i.e., rapidly cooled.
On this rapid cooling, there is no sufficient time for diffusion of Cu atoms to form the precipitate particles. Therefore a supersaturated solid solution αss (containing excess Cu) is obtained at room temperature, as shown in Fig.3.9. It should be noted that αss is not a stable structure.
Step 3: Ageing process
✓ Finally, the supersaturated solid solution αss is heated below the solvus temperature.
✓ At this ageing temperature, the diffusion of unstable αss may take place and precipitate particles can form.
✓ Then, if we hold the alloy for a sufficient time at the ageing temperature, the stable α + CuAl2 (θ) structure is produced, as shown in Fig.3.8.
✓ This fine precipitate particles of CuAl2 increase the hardness and strength of the alloy.
Note
✓ The ageing process done at room temperature is often called natural ageing. Natural ageing takes a prolonged period of time in terms of several days to reach maximum strength.
✓ Ageing at high temperature of 190°C to 260°C accelerates the precipi- tation process and the time required is reduced considerably. This process is called artificial ageing.
✓ However, natural ageing achieves maximum strength than that of the artificial ageing.
✓ Since the alloy strength develops as the alloy ages, (i.e., the strength develops with time), the precipitation hardening is also termed as age hardening.
3. Effects of Ageing Temperature and Time
✓ The effect of ageing temperature and time on the yield strength and ductility of an Al - 4% Cu alloy is illustrated in Fig.3.10 (a) and (b) respectively.
✓ From the Fig.3.10 (a), the following points can be made:
■ The strengthening process accelerates with the increase in the ageing temperature.
■ The maximum strength increases as the ageing temperature decreases.
■ After reaching a peak strength, the strength starts to de- crease. This phenomenon is called overageing.
✓ As we know, an increase in strength is associated with a reduction in ductility. This can be seen in Fig.3.10 (b).
✓ The overageing phenomenon is useful in selecting the alloys according to the required service conditions. For example, using Fig.3.10, we can understand that the aluminium age-hardened alloys are best suited for service near room temperature. Age-hardened Al-4% Cu alloy should be used at high temperatures. Because at service temperatures ranging from 100°C to 500°C, the alloy overages and loses its strength.
No comments:
Post a Comment