Copper may be alloyed with a number of elements to provide a range of useful alloys.
COPPER ALLOYS
✓ Copper may be alloyed with a number of elements to provide a range of useful alloys.
✓ The copper alloys possess a number of unique superior characteristics: High thermal and electrical conductivity, high corrosion resistance, high ductility and formability, and interesting colour for architectural uses.
✓ The important copper alloys are:
1. Brasses (copper-zinc alloys),
2. Bronzes (copper-tin alloys),
3. Gun-metals (copper-tin-zinc alloys), and
4. Cupro nickels (copper-nickel alloys).
✓ Other alloying elements and their effects on copper are as follows:
(i) Small additions of beryllium or chromium to copper give high-strength alloys.
† In recent years, the cost of copper production has increased. So for many purposes, copper has been replaced by aluminium.
(ii) A small addition of cadmium gives a significant increase in strength with little loss of electrical conductivity.
(iii) An addition of tellurium to copper gives an alloy with very good machinability
1. Brasses
✓ Brass is an alloy of copper and zinc. Sometimes, small amounts of other metals such as tin, lead, aluminium, and manganese may be added.
✓ Upto 36% zinc, brass is a single phase solid solution identified as the a phase and these alloys are called α brasses.
✓ α brasses are relatively soft, ductile, and easily cold worked.
✓ Brasses having more than 36% zinc have a two phases -- α and β phases--at room temperature. These brasses are harder and stronger than the α brasses.
Characteristics of Brasses
✓ Brasses are stronger than copper.
✓ Brasses have lower thermal and electrical conductivity than copper.
✓ They can be cast into moulds, drawn into wires, rolled into sheets, and turned into tubes.
✓ Very often 1 to 3% of lead is added to brass for improving its machining properties.
✓ The colour of brasses range from reddish colour to nearly white depending on the amount of zinc present.
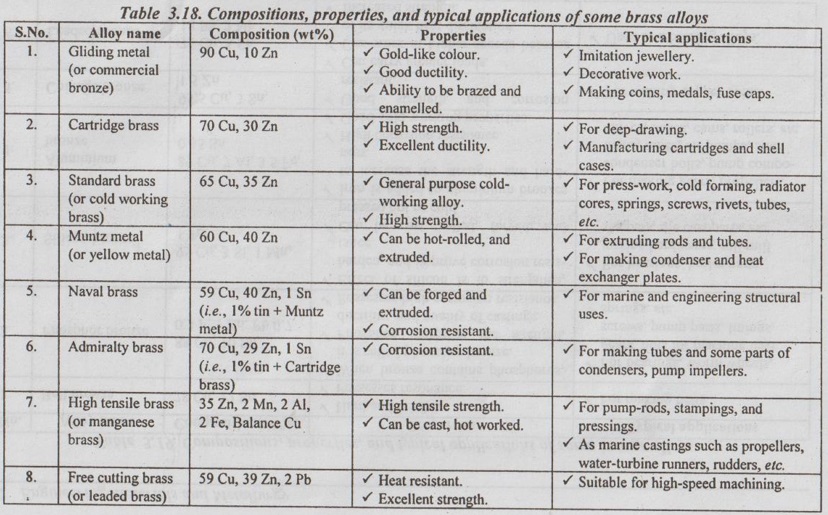
Types of Brasses
There are various types of brasses, depending upon the proportion of copper and zinc Table 3.18 presents composition, properties, and applications of some commonly used brasses.
† Though silicon bronze does not contain tin, it is also called bronze because of its colour.
2. Bronzes
✓ Bronze is an alloy of copper and tin.
✓ The bronzes are high-strength alloys with a good corrosion resistance than brasses.
✓ The strength of the bronze increases with increase in tin content. However, tin content is kept below 12% because they tend to be brittle.
✓ Bronzes can be shaped or rolled into wires, rods, and sheets.
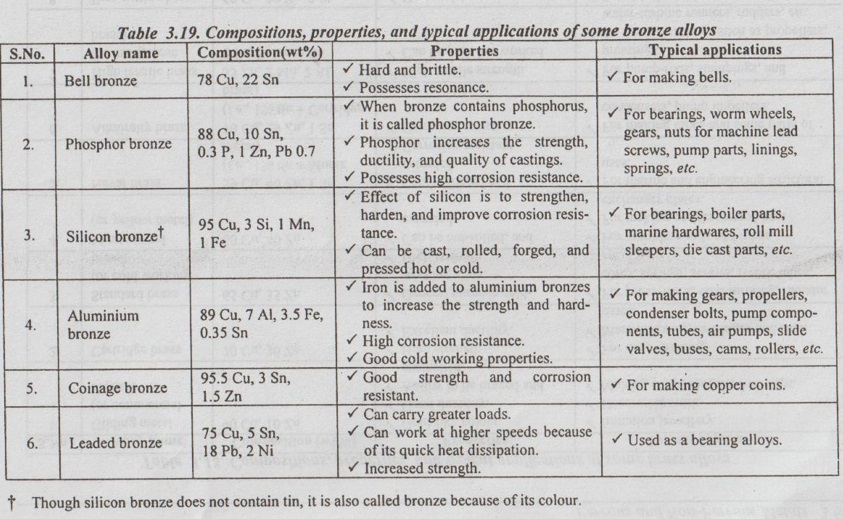
Types of Bronzes
Table 3.19 presents different types of bronzes, their compositions, properties, and typical applications.
3. Gun Metals
✓ Gun metals are alloys of copper, tin, and zinc.
✓ The zinc acts as a deoxidiser and it also improves fluidity during casting.
✓ A small amount of lead may be added to improve castability and machinability.
✓ Since zinc is considerably cheaper than tin, the total cost of the alloy is reduced.
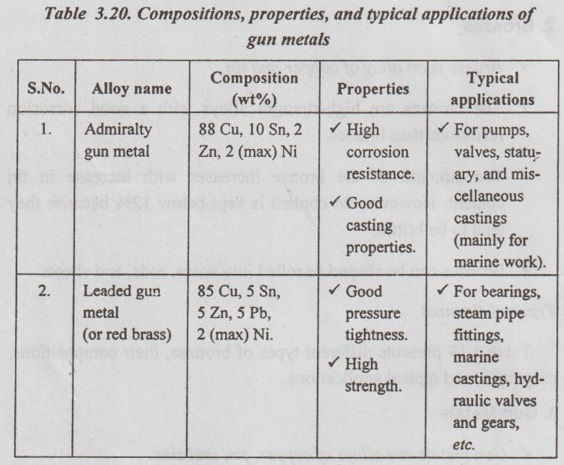
Types of Gun Metals
Table 3.20 presents the two important types of gun metals, and their compositions, properties, and typical applications.
4. Cupronickels
✓ Cupronickels are alloys of copper and nickel.
✓ The metals copper and nickel mix in all proportions in the solid state. That is, a copper-nickel alloy of any composition consists of only one phase-a uniform solid solution. Thus all copper- nickel alloys are relatively ductile and malleable.
✓ They have better corrosion resistance than many other copper alloys in sea water.
✓ They can be hot-worked or cold-worked.
✓ They can be shaped by rolling, forging, pressing, drawing, and spinning.
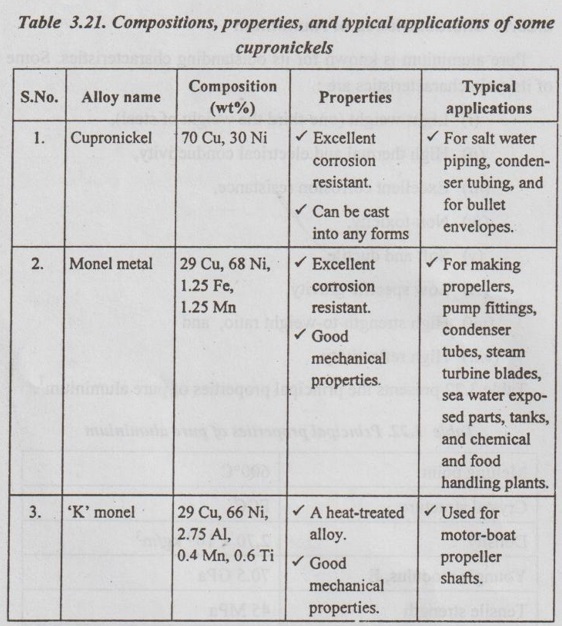
Types of Cupronickels
Table 3.21 presents some important types of cupronickels, their compositions, properties, and typical applications.
Note
The another important copper alloy is a nickel silver. Nickel silver, also known as german silver, is an alloy of Cu, Ni, and Zn. The main use of nickel silver alloys is the manufacture of cutlery and decorative articles.
No comments:
Post a Comment