1. What is mean by cyclic heat engine?
A heat engine is a device which is used to convert the thermal energy into mechanical energy.
2. What is a thermal energy reservoir? Explain the term 'source' and 'sink'.
Thermal energy reservoir:
The part which supplies or receives the heat continuously without change in its To temperature is called reservoir.
Source:
The part which supplies the heat to work absorbing or work developing device is called source.
Sink:
The part which receives the heat from work absorbing or working developing device is called sink.
3. What is a reversed heat engine?
A reversed heat engine is a device which is used to convert the thermal energy into mechanical energy.
4. Define the term COP.
Coefficient of performance is defined as the ratio of heat extracted or rejected to work input.
COP = Heat extracted/Heat rejected / Work input
5. An inventor claims have to develop an engine which absorbs 100 kW of heat from a reservoir at 1000 K produces 60 kW of work and rejects heat to a reservoir at 500 K. Will you advise investment in its development?
Given data:
Q = 100 kW
T1 = 1000 K
W = 60 kW
T2 = 500 K
Solution:
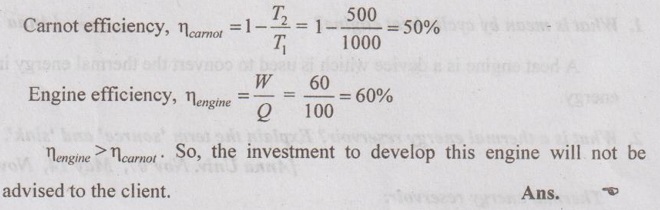
6. A heat engine with a thermal efficiency of 45 percent rejects 500 kJ/kg of heat. How much heat does it receive?
Given data:
η = 45%
Q2 = 500 kJ/kg
Solution:

7. What is the function of refrigerator? How is its COP defined?
Refrigerator is a device which is used to remove heat from a cold system. In other words, it is used to maintain the temperature of the body lower than the surroundings. Example: Air conditioners, coolers, freezers etc.
COP of a refrigerator is given by
(COP)ref = Heat extracted / Work input
8. Draw a schematic of a heat pump.
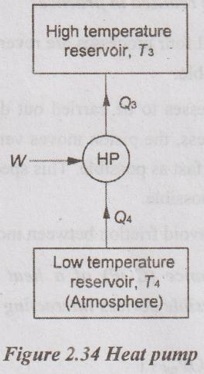
9. What is difference between a heat pump and refrigerator?
Heat pump is a device which is operated in a cyclic process by maintaining the temperature of a hot body higher than the temperature of surrounding.
A refrigerator is a device which operating in a cycle process maintains the temperature of a cold body lower than the temperature of surrounding.
10. Write the expression for COP of a heat pump and a refrigerator.

11. Deduce the relation between COP of heat pump and refrigerator.
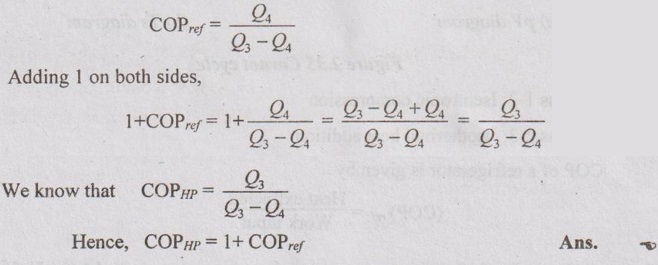
12. Why Carnot cycle cannot be realized in practice?
(i) In a Carnot cycle, all four processes are reversible but in actual practice, there is no process is reversible.
(ii) There are two processes to be carried out during compression and expansion. For isothermal process, the piston moves very slowly and for adiabatic process the piston moves as fast as possible. This speed variation during the same stroke of the piston is not possible.
(iii) It is not possible to avoid friction between moving parts completely.
13. The coefficient of performance (COP) of a heat pump is 5. Find the COP of a refrigerator if both are reversible devices interacting between same source temperature and sink temperature.
COPHP = COPref + 1
⸫ COPref = COPHP – 1 = 5 – 1 = 4 Ans.
14. Sketch the p-V and T-s diagram for Carnot cycle.
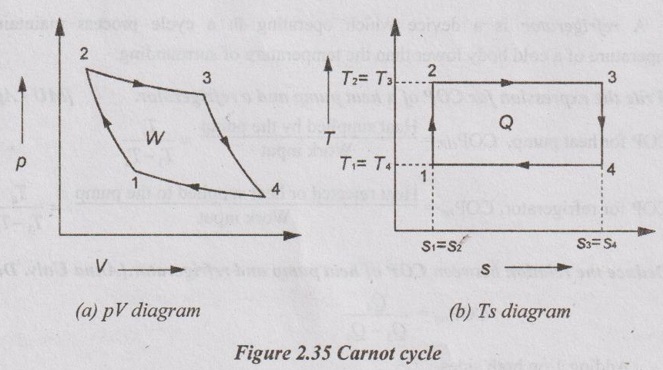
Process 1-2: Isentropic compression
Process 2-3: Isothermal heat addition
Process 3-4: Isentropic expansion and
Process 4-1: Isothermal heat rejection.
15. Name two alternative methods by which the efficiency of a Carnot cycle can be increased.
(i) Efficiency can be increased as the high temperature T2 increases
(ii) Efficiency can be increased as the low temperature T1 decreases.
16. State Carnot's theorem.
No heat engine operating in a cyclic process between two-fixed temperatures can be more efficient than a reversible engine operating between same temperature limits.
17. What are the Corollaries of Carnot theorems?
(i) All the reversible engines operating between two given thermal reservoirs with fixed temperature have the same efficiency.
(ii) The efficiency of any reversible heat engine operating between two reservoirs is independent of the nature of the working fluid and depends only on the temperature of the reservoirs.
18. Why cannot a heat engine have 100% efficiency?
For all heat engines, there will be a heat loss between system and surroundings. Therefore, the entire heat supplied to the engine cannot be converted into useful work.
19. When the Carnot cycle efficiency will be maximum?
Carnot cycle efficiency is maximum when the initial temperature is 0 K.
20. Write the expression for efficiency of the Carnot cycle.
ΗCarnot = T1 - T2 / T1
21. A reversible heat engine operates between a source at 800°C and a sink at 30°C. What is the least rate of heat rejection per kW network output of the engine?
Given data:
T1 = 800°C = 800 + 273 = 1073 K
T2 = 30°C = 30 + 273 = 303 K
Solution:

22. A reversible heat engine is operated with an efficiency of 25%. If it is operated as reversible refrigerator between the same temperature limits, what is its COP?
Solution:
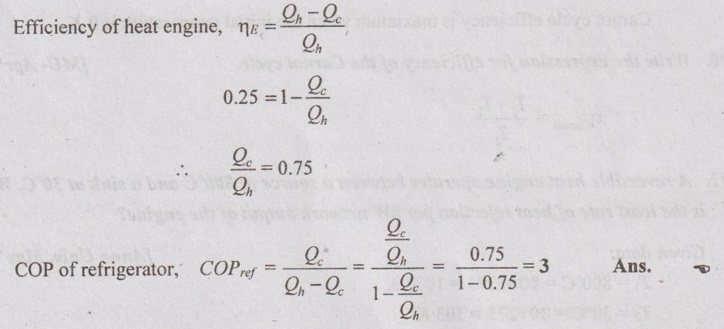
23. State the Kelvin-Planck statement of second law of thermodynamics.
Kelvin-Plank states that "it is impossible to construct an engine working on a cyclic process which converts the entire heat energy supplied to it into equivalent amount of useful work".
24. State Clausius statement of second law of thermodynamics.
Clausius statement states that heat can flow from hot body to cold body without any external aid but heat cannot flow from cold body to hot body without any external aid.
25. Why is the second law of thermodynamics called a directional law of nature?
As per Clausius statement of second law of thermodynamics, heat can flow from hot body to cold body without any external aid but heat cannot flow from cold body to hot body without any external aid. This statement helps us to identify the proper direction of flow of energy (here, thermal energy). So the second law goes by the terminology 'directional law of nature'.
26. Define - PMM of second kind.
A perpetual motion machine of second kind draws heat continuously from single reservoir and converts it into equivalent amount of work. Thus, it gives 100% efficiency.
27. Is the second law independent of first law? Explain.
Yes. The second law is essentially different from the first law; the two principles A are independent and cannot in any sense be deduced from one another. The second law speaks about the quality of energy.
28. Define entropy.
Entropy is an index of unavailability or degradation of energy. It can also be stated, it is a measure of molecular disorder or random function of a system process. It can be created but it cannot be destroyed. Heat always flows from hot body to cold body and thus it becomes less value. This unavailability of energy is measured by entropy.
29. Define change of entropy. How is entropy compared with heat transfer and absolute temperature?
The measure of irreversibility when the energy transfer takes place within the system or between system and surrounding is called change of entropy. It is simply known as unaccounted heat loss.
Heat transfer along the isotherm steps of the Carnot cycle was found to be proportional to the temperature of a system (known as its absolute temperature). This relationship was expressed in increments of entropy equal to the ratio of incremental heat transfer divided by temperature.
30. Why the performances of refrigerator and heat pump are given in terms of COP and not in terms of efficiency?
The performance of any device is expressed in terms of efficiency for work producing machines. But heat pump and refrigerator are work absorbing machines. So, the performances of those devices are based on COP only.
31. Why does the entropy of actual universe always increase?
Entropy is defined mathematically as s = dQ/dt. In our solar system, heat is generated everywhere including solar fusion and radiated heat from planets. Therefore, dQ is always a positive number. This is the reason that in our known universe, entropy is always positive.
32. Comment on the statement "The entropy of universe tends to be maximum".
Summarizing the first and second law of thermodynamics, Clausius made two statements:
1) The energy of the world (universe) is constant.
2) The entropy of the world tends towards a maximum.
Entropy is a measure of order or, most likely, disorder in the system. An irreversible process always tends to take the isolated system to a state of greater disorder. An isolated system always tends to a state of greater entropy. The entropy of the system never reduces. In actual practice the processes in all systems in the universe are irreversible. Hence, the entropy of system and universe always increases.
33. Write down the equation for Carnot COP of a heat pump which works between two heat reservoirs of temperature T3 and T4 if T3 > T4.
Carnot COP of heat pump = T3 / T3 - T4
34. What do you mean by "Calusius inequality"?
It is impossible for a self-acting machine working in a cyclic process unaided by any external agency to make the flow of heat from a body at a low temperature to a body at a high temperature.
35. Express Clausius inequality for various processes.
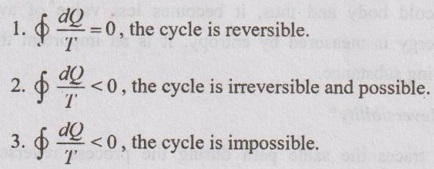
36. How do the values of the integrals 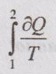 do? Compare for a reversible and irreversible process between the same end states.
do? Compare for a reversible and irreversible process between the same end states.
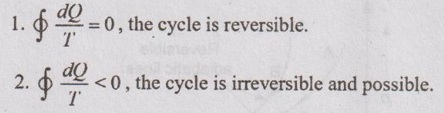
37. For compression process between same end states, which work will be more, reversible or irreversible?
Irreversible work will be more in the compressor. Generally, the actual work needed for compression will be more than the calculated work (Wrev).
38. What is the difference between adiabatic and isentropic processes?
Adiabatic process is a non-reversible process but isentropic process is a reversible process. The reversible adiabatic process is called isentropic process. The net entropy of adiabatic process is greater than zero but it is zero for reversible process.
39. A heat pump pumps 10 MJ/kWhr to the high temperature reservoir. What is COP?
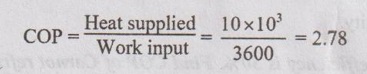
40. Find the entropy of universe when 1000 kJ of heat is transferred from 800 K to 500 K.
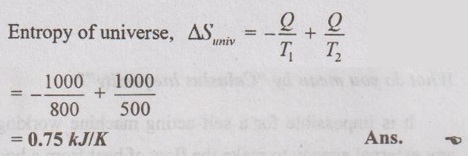
41. What do you understand by the concept of entropy?
Entropy is an index of unavailability or degradation of energy. Heat always flows from hot body to cold body and thus, it becomes less value of availability. The unavailability of energy is measured by entropy. It is an important thermodynamics property of the working substance.
42. Explain the term "Reversibility"
If the process traces the same path during the process reversed, it is called reversibility.
43. "Two reversible adiabatic lines cannot intersect". Is this statement true or false? Justify the answer.
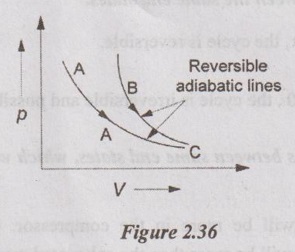
Yes. It is true because entropy is constant throughout the process. So, constant property lines never can intersect with each other. If it is so, it will violate Kelvin-Planck statement. So, there can only pass one reversible adiabatic through one point as shown in Figure 2.36.
44. Can entropy of universe ever decrease? Why?
Entropy of universe cannot ever decrease. It will remain constant or will increase due to irreversibility.
45. If Carnot engine efficiency is 50%. Find COP of Carnot refrigerator working between same temperatures.

46. What is meant by thermodynamic temperature scale? How do you device such scale?
A temperature scale which is independent of the properties of the substances that are used to measure temperature is called a thermodynamic temperature scale.
Thermodynamic temperature is defined by the third law of thermodynamics in which the theoretically lowest temperature is the null or zero point. The International System of Units specifies a particular scale for thermodynamic temperature. It uses the Kelvin scale for measurement. The temperature measured on this scale is known as absolute temperature. The magnitude of a Kelvin is defined as 1/273.16 of the temperature interval between absolute zero and triple- point temperature of water.
47. What is meant by dead state?
The state of a system when it is in thermodynamic equilibrium with its environment is called dead state. If the system is in a state different than the dead state, it can always produce more work. Normally, the dead state is taken to be our environment. For many cases, it is taken as 25°C, 101.325 kPa. At dead state:
1. Zero velocity relative to the environment
2. At the lowest elevation in the environment - ground level.
The properties of system at dead state are: To, po, uo, ho and so.
48. Define entropy of a pure substance.
Entropy is an index of unavailability or degradation of energy. It can also be stated, it is a measure of molecular disorder or random function of a system process. It can be created but it cannot be destroyed. Heat always flows from hot body to cold body and thus it becomes less value. This unavailability of energy is measured by entropy.
No comments:
Post a Comment