Entropy is an index of unavailability or degradation of energy.
CONCEPT OF ENTROPY
Entropy is an index of unavailability or degradation of energy. It can also be stated, it is a measure of molecular disorder or random function of a system process. It can be created but it cannot be destroyed. Heat always flows from hot body to cold body and thus it becomes less value. This unavailability of energy is measured by entropy. It is an important thermodynamic property of the working substance. It increases with the addition of heat and decreases with its removal. It is the function of quantity of heat with respect to temperature.
We are usually interested in change in entropy. The change in entropy for reversible process is mathematically given by
Change in entropy, dS = Change of heat transfer / Absolute temperature = dQ / T
Unit of entropy is kJ/K or J/K
The change in entropy of a system during a process can be determined by integrating the above equation between initial and final states:
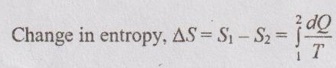
Note:- For reversible adiabatic process, the change in entropy is zero.
No comments:
Post a Comment