It is also called constant temperature cycle.
CARNOT CYCLE AND ITS PERFORMANCE
It is also called constant temperature cycle. This cycle was introduced by Sadi Carnot. It consists of four processes such as two isentropic or reversible adiabatic and two isothermal processes. p-V and T-s diagrams for Carnot cycle are given in Figure 2.9.
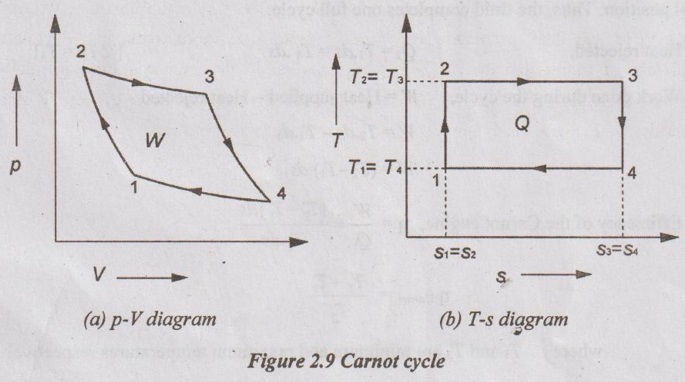
Process 1-2: Isentropic compression
Air is isentropically compressed from state 1 to state 2.
During this process, both pressure and temperature increase from p1 to p2 and T1 to T2 respectively. But, the volume decreases from V1 to V2. There is no heat addition or rejection during this process. Therefore, the entropy remains constant (s1 = s2).
Process 2-3: Isothermal heat addition
During this process, the heat is supplied to the fluid at constant temperature. Therefore, there is no change in temperature i.e. T3 = T2 but both volume and entropy increase from V2 to V3 and s2 to s3 respectively. Also, the pressure decreases from p2 to p3.
Heat supplied, Q1 = T2.ds = T3.ds
⸫ ds = dQ/T and T = T2 = T3
dQ = T.ds
Process 3-4: Isentropic expansion
Air is isentropically expanded from state 3 to state 4. During this process, both pressure and temperature decrease from p3 to p4 and T3 to T4 respectively. But, the entropy remains constant (s3 = s4).
Process 4-1: Isothermal heat rejaction
During this process, the heat is isothermally rejected from the fluid and it attains its initial position. Thus, the fluid completes one full cycle.
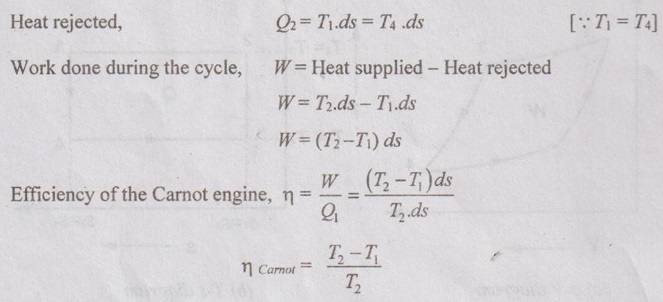
where T1 and T2 are minimum and maximum temperatures respectively.
In this cycle, all four processes are reversible. Therefore, it is a reversible cycle. By Carnot theorem, the reversible engine gives the maximum efficiency than any other engine. Thus, the Carnot cycle produces maximum efficiency than any other cycle.
No comments:
Post a Comment