As all the processes in the Carnot cycle are reversible, it can be reversed. In this case, it becomes the Carnot refrigeration cycle.
REVERSED CARNOT CYCLE AND ITS PERFORMANCE
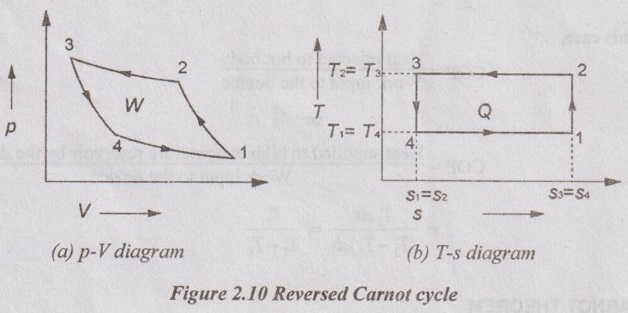
As all the processes in the Carnot cycle are reversible, it can be reversed. In this case, it becomes the Carnot refrigeration cycle. This cycle is used to extract heat from cold body and reject it into hot body (atmosphere). This cycle consists of two isothermal and two isentropic processes. p-V and T-s diagrams of reversed Carnot cycle are shown in Figure 2.10.
Process 1-2: Isentropic compression in a compressor
Process 2-3: Isothermal heat rejection to a hot body
Process 3-4: Isentropic expansion in a expansion valve
Process 4-1: Isothermal heat extraction from a cold body.
The processes isentropic compression and expansion are similar to Carnot cycle.
Heat rejection to the hot body during 2-3 (Condenser part of the refrigerator):
Heat rejected, Q3 = T3.ds = T2.ds
Heat extraction from the cold body during process 4-1 (Evaporator part of refrigerator):
Heat extraction, Q4 = T4.ds = T1 .ds
Work input, W = Q3 – Q4
= T3.ds - T4.ds = (T3 - T4) ds
This machine is used to produce cooling effect. Therefore, the term "Coefficient Of Performance (COP)" is used. It is defined as "the ratio of heat extracted to the work input".
COP = Heat extraction from cold body / Work input to the device = Q4 / W

where T4 and T3 are minimum and maximum temperatures respectively.
The above equation is used for refrigerators.
Reversed Carnot cycle for Heat pump:
If the same cycle is operated as a heat pump, the heat will be extracted from low temperature (atmosphere) and supplied to it into higher temperature (room) for the purpose of heating.
In this case,

No comments:
Post a Comment