The statements which can be proved with the help of reversible cycles are called corollaries of the second law of thermodynamics.
COROLLARIES OF THE SECOND LAW OF THERMODYNAMICS
The statements which can be proved with the help of reversible cycles are called corollaries of the second law of thermodynamics.
Corollary - 1
It is impossible to construct a system which will operate in a cycle and transfer the heat from a cold body to a hot body without supplying work on the system by surroundings.
Proof:
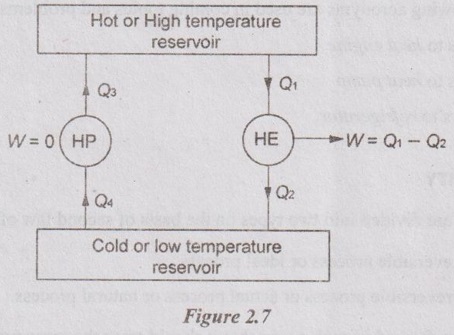
If above-said statement is not true, the system could be represented by a heat pump for which W = 0 as shown in Figure 2.7. If it takes Q4 units of heat from the cold reservoir, it must deliver Q4 = Q3 units to the hot reservoir to satisfy the first law.
A heat engine could also be operated between two reservoirs. It delivers Q2 units of heat to the cold reservoir while performing W units of work. Then, the first law states that the engine must be supplied with (W+Q2) units of heat from the hot reservoir.
If both plants are combined, then the cold reservoir becomes unnecessary and the heat rejected from heat engine is directed to heat pump as heat input. The combined plant represents a heat engine extracting (W+Q2) - Q2 = W units of heat from a reservoir and delivering an equivalent amount of work. It is impossible according to second law. Thus, corollary -1 must be true.
Corollary - 2
An engine operating between two heat reservoirs cannot have higher efficiency than a reversible engine operating between same temperature limits of heat reservoirs.
Corollary - 3
All reversible engines operating between same two reservoirs have the same efficiency.
Corollary - 4
The efficiency of any reversible engine operating between more than two reservoirs must be less than the reversible engine operating between two reservoirs which have temperatures equal to the highest and lowest temperatures of the fluid in the original engine.
Corollary - 5
Whenever a system undergoes a cycle, 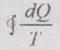 will be zero if the cycle is reversible and it is negative if the cycle is irreversible, i.e. in general
will be zero if the cycle is reversible and it is negative if the cycle is irreversible, i.e. in general ![]() ≤ 0.
≤ 0.
Corollary - 6
The entropy of any closed system which is thermally isolated from surroundings remains constant. If a process undergone by the system is reversible then the entropy remains constant.
Perpetual Motion Machine of Second Kind (PMM-II):
PMM-II is the machine which receives heat energy from hot reservoir and converts it into equivalent amount of work i.e., PMM-II gives 100% efficiency. Therefore, it is impossible to construct. It violates the second law of thermodynamics.
Note:
The following acronyms are used in coming topics and problems in our book.
HE refers to heat engine
HP refers to heat pump
Ref. refers to refrigerator.
No comments:
Post a Comment