1. What is a pure substance? Give examples.
Pure substance is a substance which has a fixed chemical composition throughout its mass. Examples: Water, nitrogen, carbon dioxide and helium. A pure substance does not have to be of a single chemical element or compound. A mixture of various chemical elements or compounds is also called pure substance as long as the mixture is homogeneous.
2. What is saturation temperature and saturation pressure?
At a given pressure, the temperature at which a liquid boils is called saturation temperature. At the given temperature, the pressure at which the liquid boils is called saturation pressure. It is also called vapour pressure.
3. What is meant by superheated steam? and indicate its use.
If the dry steam is further heated, then the process is called superheating and the steam is known as superheated steam.
Uses:
1. Superheated steam has more heat energy and more work can be obtained using it.
2. Thermal efficiency increases as the temperature of superheated steam is high.
3. Heat losses are due to condensation of steam and cylinder wall friction.
4. Define latent heat of evaporization.
The amount of heat added during heating of water from boiling point to dry saturated state is called latent heat of vaporization or enthalpy of vaporization or latent heat of steam.
5. Define the terms 'Boiling point' and 'Melting point'.
Boiling point:
It is the temperature at which the liquid starts to change its phase from liquid to vapour.
Melting point:
It is the temperature at which the solid starts to change its state from solid to liquid.
6. Find the saturation temperature and latent heat of vaporization of steam at 1 MPa.
From saturated water of pressure scale, corresponding to 1 MPa or 10 bar,
Saturation temperature, Tsat = 179.88°C
Latent heat of vaporization, hfg = 2013.6 kJ/kg
7. Define: sensible heat of water.
Sensible heat of water is defined as "the amount of heat required to raise the temperature of unit mass of water from 0°C to the saturation temperature under a constant pressure." It is denoted by hf.
8. Define the term "super heat enthalpy".
The heat supplied to the dry steam at saturation temperature to convert it into superheated steam at the temperature Tsup is called superheat heat enthalpy.
9. What are wet and dry steams?
The steam which is partially evaporated and having water particles in suspension is called wet steam. The steam which is completely in evaporated state without any water particles is called dry steam.
10. Is iced water a pure substance? Why?
Yes. Iced water is a pure substance. The chemical composition will be same even the phase changes.
11. Define dryness fraction of steam.
Dryness fraction is defined as the ratio of the mass of the total steam actually present to the mass of the total mixture steam.
Dryness fraction = Mass of dry steam / Mass of total mixture
12. Explain the use of Throttling Calorimeter to determine dryness fraction.
This calorimeter is more suitable for finding dryness fraction of steam having very low portion of wet particles. It means, the steam is with high dryness fraction. It is well known that the total enthalpy remains constant before and after throttling process.
13. Why are the temperature and pressure dependent properties in the saturated mixture region?
The temperature cannot be varied while holding the pressure constant. When the pressure changes, the temperature will also vary.
14. Explain the terms: Degree of super heat and degree of subcooling.
Degree of super heat:
It is the difference between superheated temperature and saturated temperature at the same pressure.
Degree of subcooling:
It is the amount by which the water is cooled beyond its saturated temperature at the same pressure.
15. Define triple point and critical point for pure substance.
Triple point:
Triple point is the state at which all three phases i.e. solid, liquid and vapour exists in equilibrium.
Critical point:
It represents the highest pressure and temperature at which the liquid and vapour phases coexist in equilibrium. At the critical point, the liquid and vapour phases are indistinguishable, i.e. liquid is directly converted into vapour.
16. What is triple point? For a pure substance, how many degrees of freedom are there at triple point?
Triple point is the state at which all three phases i.e. solid, liquid and vapour exist in equilibrium.
The number of independent variables associated with a multi-component, multiphase system is given by the phase rule. It is also called Gibbs phase rule. It is expressed by
n = C – ϕ + 2
where
n = Number of independent variables
C = Number of components
ϕ = Number of phases present in equilibrium.
For pure substance, the water (C = 1) at the triple point has 3 phases (ϕ = 3).
Degree of freedom, n = C – ϕ + 2 = 1 – 3 + 2 = 0
17. What is critical condition of steam?
At a particular point, the liquid is directly converted into vapour without forming the liquid-vapour mixture. This point is called critical point or critical condition of steam. It represents the highest pressure and temperature at which the liquid and vapour phases coexist in equilibrium. At the critical condition, the liquid and vapour phases are indistinguishable, i.e. liquid is directly converted into vapour.
The critical pressure, temperature and volume of water are 221.2 bar, 374.15°C and 0.00317 m3/kg respectively.
18. Define critical pressure and temperature for water.
The pressure at which the water is directly converted into vapour without formation of liquid-vapour mixture is known as critical pressure. The corresponding temperature is known as critical temperature.
Critical pressure, pcr = 221.2 bar
19. When saturation pressure increases, what happens to saturation temperature and freezing point?
When the saturation pressure increases, then the saturation temperature is increased and the freezing point is reduced.
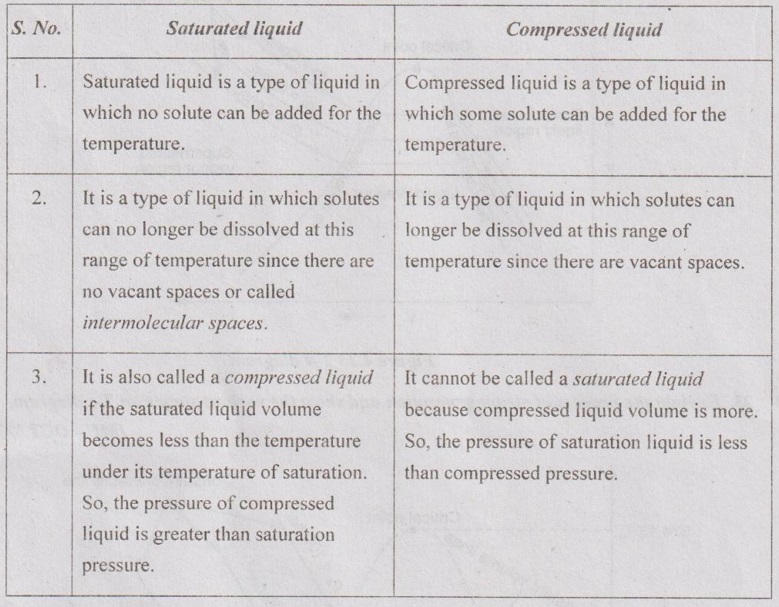
20. Differentiate between saturated liquid and compressed liquid.
21. List the advantages in superheating of steam.
1. Superheated steam has more heat energy and more work can be obtained using it.
2. Thermal efficiency increases as the temperature of superheated steam is high.
3. Heat losses are due to condensation of steam and cylinder wall friction.
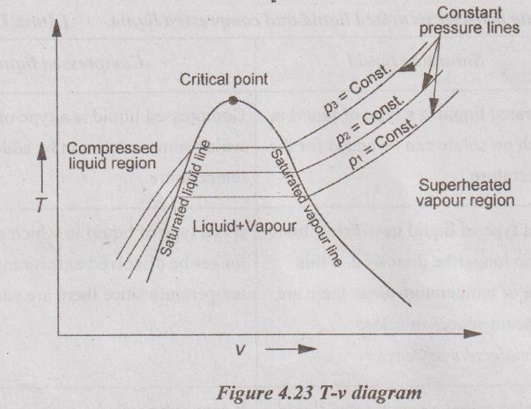
22. Explain the change of phase of water in T-v diagram.
1-2 ⇒ Solid stage
2-3 ⇒ Melting stage
3-4 ⇒ Liquid stage
4-5 ⇒ Vapourising stage
5-6 ⇒ Superheating stage.
23. Explain the process of steam generation and show the various stages on T-s diagram.
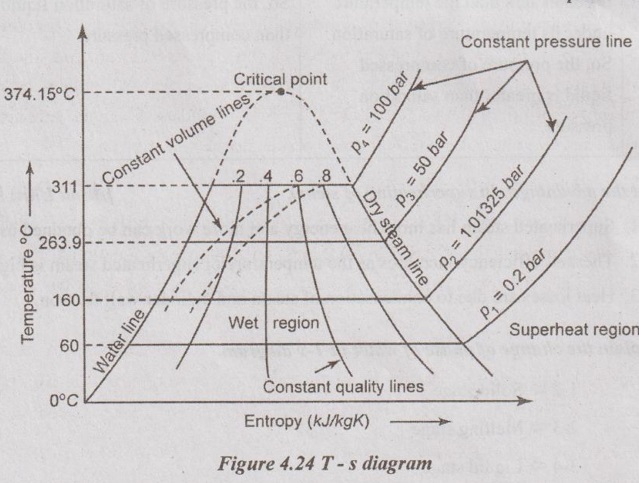
In T-s diagram, the region left of the water line, the water exists as liquid. In the right of the dry steam line, the water exists as a superheated steam. In between water and dry steam line, the water exists as a wet steam. Therefore, the dryness fraction lines are represented in this region. The value of various quantities can be directly read from the diagram. From Figure 4.24, the water line and steam line are converging with the increase in temperature. At a particular point, the water is directly converted into dry steam without forming the wet steam. This point is called critical point.
24. Draw a p-v-T surface of water and also indicate its salient features.
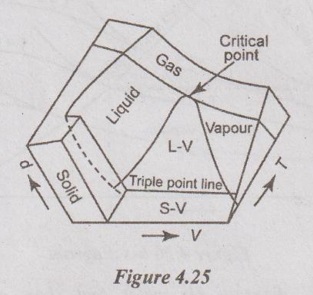
The three important thermodynamic properties such as pressure (p), specific volume (v) and temperature (T) are plotted in a three dimensional coordinate. This plot is called p-v-T surface. Here, T and v may be viewed as the independent variables (the horizontal surface) and p as the dependent variable (the vertical surface). All points on the surface represent equilibrium states. The single phase region appears as curved surfaces on p-v-T surface and two phase regions as surfaces perpendicular to p-T plane.
25. Write a short note on Mollier chart.
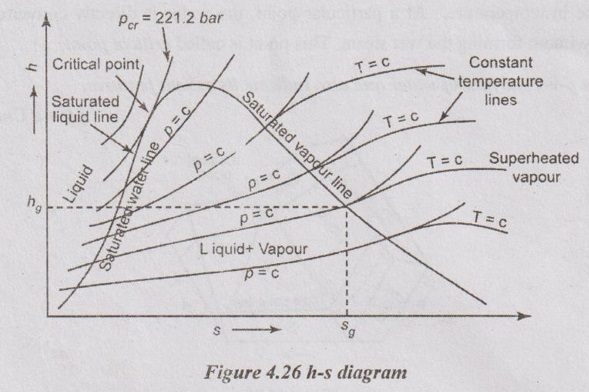
In Mollier chart (h-s diagram), the vertical ordinate represents the enthalpy while the base represents the entropy. In h-s diagram, the dry steam line divides this chart into two regions. The region which is below the dry steam line represents the wet condition of steam. Here, the dryness fraction lines are shown parallel to dry steam line. The region above the dry steam line represents the superheated condition of steam. In this region, the constant temperature lines are drawn. At the same time, the lines at constant pressure are straight in the wet steam region but it is curved in the superheated region.
26. What is the latent heat of evaporation?
The amount of heat added during heating of water from boiling point to dry saturated state is called latent heat of vaporization or enthalpy of vaporization or latent heat of steam.
27. Draw a h-s diagram for steam and show a throttling process on it.
28. Draw a skeleton p-V diagram for water and show an isotherm passing from compressed liquid state to superheated vapour state through vapourisation process.
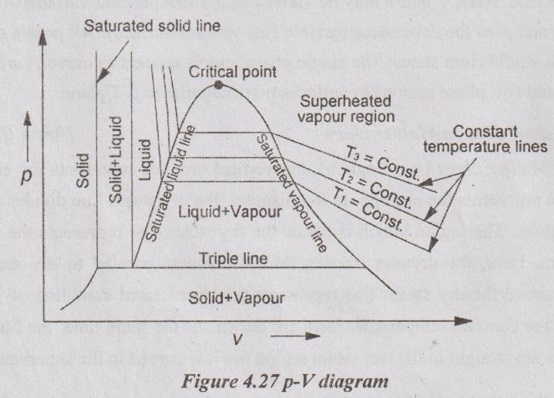
29. Write the formula for calculating entropy change from saturated water to superheat steam condition.
Entropy of superheated steam, ssup = sg + Cp In(Tsup/T)
Where sg - Entropy of dry steam
Tsup - Superheated temperature
Ts - Saturated temperature
Cp - Specific heat of superheated steam.
30. Determine the condition of steam of 2 bar whose entropy is 6.27 kJ/kg.
From saturated water table of pressure scale, corresponding to 2 bar,
sg = 7.1268 kJ/kgK
Since entropy of given steam of pressure 2 bar is less than the entropy of dry steam at that pressure, the steam is in wet condition.
31. Determine specific enthalpy and specific entropy of 120°C saturated steam.
From saturated water table of temperature scale, corresponding to 120°C,
Specific enthalpy, hg = 2706 kJ/kg
Specific entropy, sg = 7.1293 kJ/kgK
32. Draw a p-T(pressure-temperature) diagram for a pure substance.
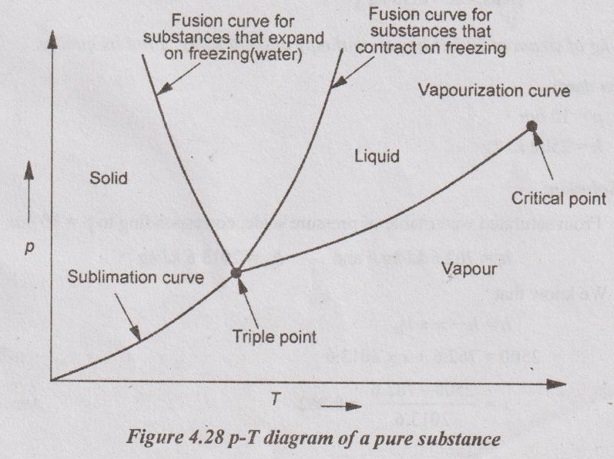
33. Find the mass of 0.1 m3 of wet steam at a temperature of 160° and 0.94 dry.
Given data:
V = 0.1 m3
T = 160°C
x = 0.94
Solution:
From saturated water table of temperature scale, corresponding to 160°C,
vf = 0.001102 m3/kg and vg = 0.307 m3/kg
Specific volume of wet steam = vf + x × vfg
= 0.001102 + 0.94 × 0.307 = 0.29 m3/kg
Mass of steam, m = Volume of given wet steam / Specific volume of wet steam
= 0.1/0.29 = 0.345 kg Ans.
34. Find the mass of 0.7 m3 of wet steam at 150°C and 90% dry.
Similar to Question 28 on Page 4. 204.
[Ans:- m = 1.98 kg]
35. One kg of steam at 10 bar has an enthalpy of 2500 kJ/kg. Find its quality.
Given data:
p = 10 bar
h = 2500 kJ/kg
Solution:
From saturated water table of pressure scale, corresponding to p = 10 bar
hf = 762.6 kJ/kg and hfg = 2013.6 kJ/kg
We know that
h = hf + x × hfg
2500 = 762.6 + x × 2013.6
x = 2500 - 762.6 / 2013.6
= 0.862 Ans.
36. Determine whether water at the following states is a compressed liquid, a superheated vapour or a mixture of saturated water steam. (a)18 MPa, 0.003 m3/kg (b) 130°C, 200 kPa.
Case (a):
p = 18 MPa
v = 0.003 m3/kg
From saturated water table of pressure scale, corresponding to p = 18 MPa = 180 bar,
vg = 0.007497 m3/kg
Since v < vg, the steam is in wet condition i.e. mixture of liquid and steam.
Case (b):
T = 130°C.
p = 200 kPa
From saturated water table of pressure scale, corresponding to p = 200 kPa = 2 bar,
Tsat = 120.2°C
Since T < Tsat, the steam is in superheated condition.
37. If water is at 65°C at 1 atm, what is the state of water? What is its specific enthalpy?
From saturated water table of temperature scale, corresponding to 65°C, saturation pressure,.
psat = 25.03 kPa
Since psat < p, the water is steam which is in superheated state. Ans.
Specific enthalpy, h = 2621.07 kJ/kg Ans.
38. A vessel of 2 m3 contains a wet steam of quality 0.8 at 210°C. Determine the mass of the liquid and vapour present in the vessel.
Given data:
V = 2 m3
T = 210°C
x = 0.8
To find:
Mass, m
Solution:
From saturated water table of temperature scale, corresponding to 210°C,
vf = v1 = 0.0011726 m3/kg
vg = vs = 0.1042 m3/kg
We know that v = v + xvfg
v = 0.0011726 + 0.8 (0.1042 – 0.0011726) [⸪ vfg = vg - vf]
v = 0.0836 m3/kg
⸫ Mass of steam, m = Total volume / Specific volume = V/v = 2/0.0836 = 23.923 kg
Also,
Mass of vapour in wet steam,
mg = mx = 23.923 × 0.8 = 19.14 kg Ans.
⸫ Mass of liquid in wet steam,
mf = m (1 - x) = 23.923 (1 - 0.8) = 4.785 kg Ans.
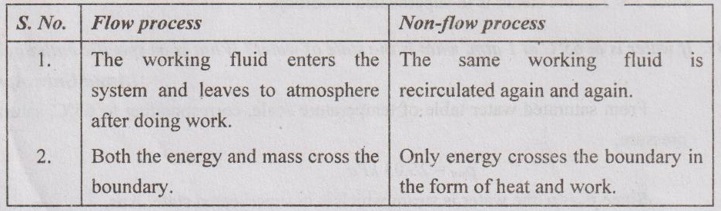
39. Distinguish between 'flow process' and 'non-flow process'.
No comments:
Post a Comment