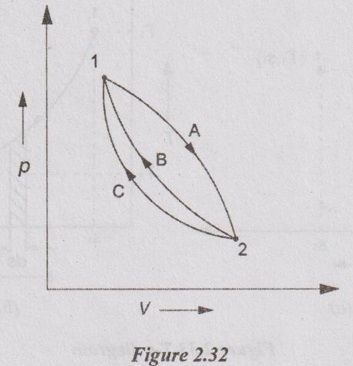
Let, a thermodynamic system undergoes a change of state from 1 to 2 by a reversible process 1-A-2 and returns to its original state 1 by another reversible process 2-B-1 and completing a cycle 1-2-1.
ENTROPY: A PROPERTY OF THE SYSTEM
Let, a thermodynamic system undergoes a change of state from 1 to 2 by a reversible process 1-A-2 and returns to its original state 1 by another reversible process 2-B-1 and completing a cycle 1-2-1.
For this cyclic reversible process, the entropy equation is given by

Now, let us consider the cycle 1-2-1 completed by another reversible process 2-C-1.
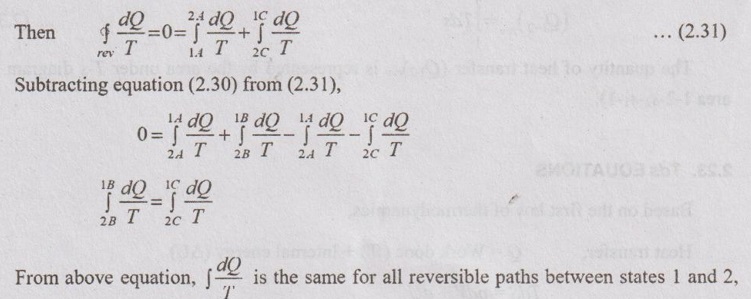
i.e., it is independent of the path and a function of end states only. Hence, the entropy is a property of a system.
No comments:
Post a Comment