Problems for Practice: Gas Mixtures and Thermodynamic Relations - Engineering Thermodynamics
PROBLEMS FOR PRACTICE
1. Compute the specific volume of steam at 1 bar and 550 K using Van der Waals equation. Take critical temperature of steam is 647.3 K and Critical pressure is 220.9 bar.
2. A vessel of volume 0.5 m3 contains 12 kg of air at 300 K. Determine the pressure exerted by the air using
1. Perfect gas equation
2. Van der Waals equation
3. Generalised compressibility chart.
Take critical temperature of air is 132.8 K and critical pressure of air is 37.7 bar.
3. The gas neon has a molecular weight of 20.183 and its critical temperature, pressure and volume are 50 K, 3 MPa and 0.08 m3/kmol. Reading from a compressibility chart for a reduced pressure of 2 and a reduced temperature of 1.2, the compressibility factor Z is 0.8. What are the corresponding specific volume, pressure, temperature and reduced volume?
4. A perfect gas of 0.3 kg has a pressure of 320 kPa, a temperature of 45°C and a volume of 0.08 m3. The gas undergoes an irreversible adiabatic process to a final pressure of 420 kPa and final volume of 0.1 m3, work done on the gas is 60 kJ. Find Cp and Cv.
5. One kg of ideal gas is heated from 60°C to 120°C. If R = 280 J/kg K and γ = 1.32 for the gas, determine:
(1) Cp and Cv
(2) Change in internal energy,
(3) Change in enthalpy,
(4) Change in flow energy.
6. The latent heat of vaporization at 1 bar pressure is 2290 kJ/kg and the saturation temperature is 95.4°C. Calculate the saturation temperature at 2.2 bar pressure using Clausius-Clapeyron equation. Verify the same from the steam table data.
7. Methane at 1.013 bar and 30°C enters an insulated mixing chamber at a rate of 5 kg/s. It is mixed with air at 1 bar in an air/methane mass ratio of 18. The flow is steady and kinetic energy changes are negligible. Ambient pressure and temperature are 1.013 bar and 30°C, determine the (a) temperature of the mixture leaving the chamber and (b) irreversibility of the mixing per kilogram of methane. Take CV and Cp of methane as 1.7354 kJ/kgK and 2.2537 kJ/kgK respectively.
8. A mixture of ideal gases consists of 10 kg nitrogen and 15 kg of carbon dioxide at a pressure 300 kPa and a temperature of 28°C. Find the
(i) mole fraction of each constituent
(ii) equivalent molecular weight of the mixture
(iii) equivalent gas constant of the mixture
(iv) volume and density of the mixture, and
(v) Cp and Cv of the mixture.
If the mixture is heated at constant volume to 40°C, find the changes in internal energy, enthalpy and entropy of the mixture. Find the same properties of the mixture if the heating is carried at constant pressure to 50°C. Take γ for N2 and CO2 as 1.4 and 1.286.
9. Find the increase in entropy when 7.5 kg of oxygen at 70°C are mixed with 8 kg of nitrogen at the same temperature. The initial pressure of each constituent is 103 kPa and is the same as that of mixture.
10. A vessel is divided into three compartments (a), (b) and (c) by two partitions. Part(a) contains oxygen and has a volume of 0.3 m3, (b) has a volume of 0.5 m3 and contains nitrogen while (c) is 0.1 m3 and holds CO2. All three parts are at a pressure of 2 bar and a temperature of 13°C. When the partitions are removed and the gases mix, determine the change in entropy of each constituent, final pressure in the vessel and partial pressure of each gas. The vessel may be taken as being completely isolated from its surroundings.
11. A closed rigid cylinder is divided by a diaphragm into two equal compartments, each of volume 0.5 m3. Each compartment contains air at a temperature of 30°C. The pressure in one compartment is 2.0 MPa and in the other compartment is 1.5 MPa. The diaphragm is ruptured so that the air in both the compartments mixes to bring the pressure to a uniform value throughout the cylinder which is insulated. Find the net change of entropy for the mixing process.
12. A mixture of gases contains 55% N2, 35% O2, and 10% CO2 by mass. 5 kg of the mixture is compressed from 200 kPa and 298 K to 450 kPa polytropically which follows the pV1.2 = C. Determine the work done, heat transferred and change in entropy. Take (Cp)N2 = 1.04kJ/kgK, (Cp)co2 = 0.918 kJ/kgK and (Cp)co2 = 0.846 kJ/kgK.
13. A mixture of perfect gases at 25°C contains 65% N2, 20% O2 and 25% CH4 by volume. If the partial of CH4 is 65 kPa, determine the (a) partial pressure of N2 and O2, (b) mass proportion of mixture, (c) gas constant for the mixture and (d) volume per mole of mixture.
14. A mixture of 7.5 kg of N2 and 8kg of O2 at a pressure of 103 kPa and 308 K is compressed reversibly and adiabatically to a pressure of 550kPa. Determine the (a) initial and final pressure of each component (b) final temperature (c) change in internal energy, enthalpy (d) entropy and (e) work done on the mixture. Take (Cp)N2 = 1.04kJ/kgK and (Cp)O2 = 0.914 kJ/kgK.
15. A mixture of 5 kg oxygen and 7.5 kg Argon is in an insulated piston cylinder arrangement at 103 kPa, 330 K. The piston now compresses the mixture to half its initial volume. Molecular weight of oxygen is 32 and for argon is 40. Ratio of specific heats for oxygen is 1.39 and for argon is 1.667.
16. 0.6 kg of CO and 1.5 kg of air is contained in a vessel of volume 0.5 m3 at 15°C. Air has 23.3% of O2 and 76.7% of N2 by mass. Calculate the partial pressure of each constituent and total pressure in the vessel. Molar masses of CO, CO2 and N2 are 28, 32 and 28kg/kmol.
17. The pressure and temperature of mixture of 4 kg of O2 and 6 kg of N2 are 4 bar and 27°C respectively. For the mixture, determine the following:
(i) mole fraction of each component
(ii) average molecular weight
(iii) specific gas constant
(iv) volume and density
(v) partial pressures and partial volumes.
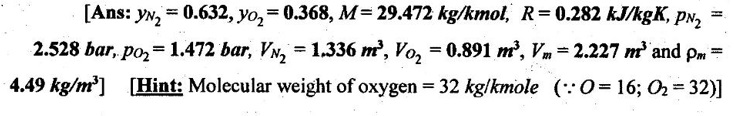
No comments:
Post a Comment