A temperature scale which is independent of the properties of the substances that are used to measure temperature is called a thermodynamic temperature scale.
ABSOLUTE THERMODYNAMIC TEMPERATURE SCALE
A temperature scale which is independent of the properties of the substances that are used to measure temperature is called a thermodynamic temperature scale. This temperature scale should be very much useful in thermodynamic calculations and its derivation using some reversible heat engines.
Based on corollaries of the second law of thermodynamics, all reversible heat engines have the same thermal efficiency when operating between same two reservoirs T1 and T2. The efficiency of a reversible heat engine is independent of the working fluid and its properties. The reservoirs are differentiated by their temperature as a cold reservoir or hot reservoir. Therefore, the efficiency of heat engines is a function of the reservoir temperatures only.
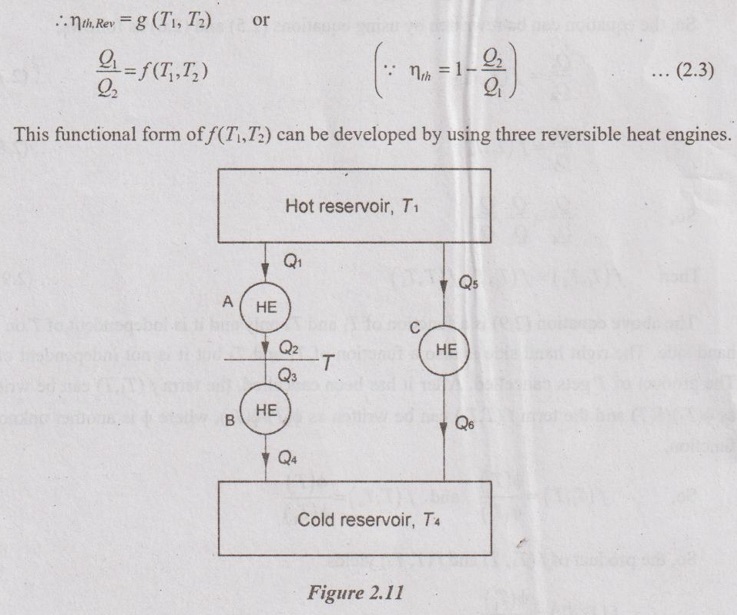
Let, the three reversible heat engines A, B and C are arranged as shown in Figure 2.11. Engine A and engine C draw the heat from T1 reservoir. Engine A rejects heat to the engine B. The engine B receives this heat from A and rejects to the low temperature reservoir, T4.
But, engine C directly rejects heat to the low temperature reservoir, T4. The intermediate temperature between engine A and B is T2 = T3 = T.
Applying the above equation (2.3) for all three engines A, B and C respectively,
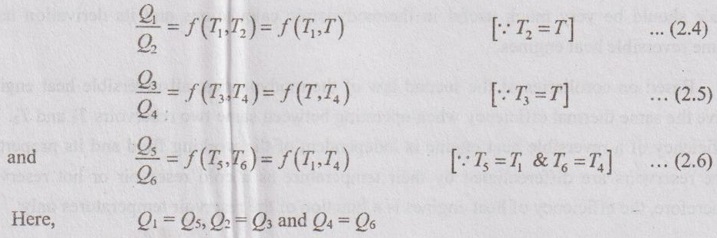
So, the equation can be rewritten by using equations (2.5) and (2.6) as follows,
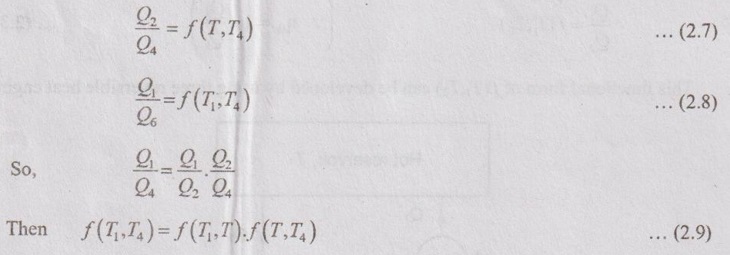
The above equation (2.9) is a function of T1 and T4 only and it is independent of T on left hand side. The right hand side is also a function of T1 and T4 but it is not independent of T. The product of T gets cancelled. After it has been cancelled, the term f (T1, T) can be written as ϕ(T1) / ϕ(T) and the term ƒ (T, T4) can be written as ϕ(T) / ϕ(T4) where ϕ is another unknown function.
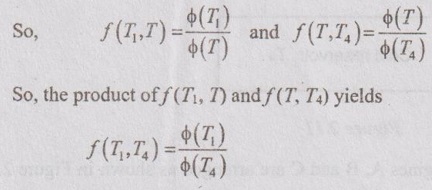
This obeys Kelvin-Planck statement of second law of thermodynamics. So, it can be written as ϕ(T) = T. Then the above equation becomes

This temperature scale is known as Kelvin scale. The temperature measured on this scale is known as absolute temperature.
The temperature scale is not completely defined. At International Conference on Weights and Measures (1954), the triple-point of water was assigned as the value of 273.16 K. The magnitude of a Kelvin is defined as 1/273.16 of the temperature interval between absolute zero and triple- point temperature of water. The magnitudes of temperature units on the Kelvin and Celsius scales are identical (1 K = 1°C). The temperatures on these two scales differ by a constant 273.16.
T(°C) = T(K) - 273.16
No comments:
Post a Comment