Stainless steels are alloys of iron, chromium, and other elements that resist corrosion from many environments.
STAINLESS STEELS
1. What are Stainless Steels?
✓ Stainless steels are alloys of iron, chromium, and other elements that resist corrosion from many environments.
✓ Stainless steels are also known as corrosion-resistant steels or chromium-bearing steels.
2. Effect of Cr on Stainless Steel
✓ All true stainless steel contain minimum of about 12% Cr. This 12% Cr permits a thin (but extremely dense) protective surface layer of chromium oxide to form when the steel is exposed to oxygen.
✓ The chromium oxide (extremely dense-thin) protective layer acts as a barrier to retard further oxidation, rust or corrosion. As this steel cannot be stained easily, it is called stainless steel.
† In contrast to stainless steel, ordinary steel is coated with a loose, porous of rust layer, through which the atmosphere can pass and cause further corrosion. For this reason, ordinary steel rusts quickly and thus it is less corrosion resistant.
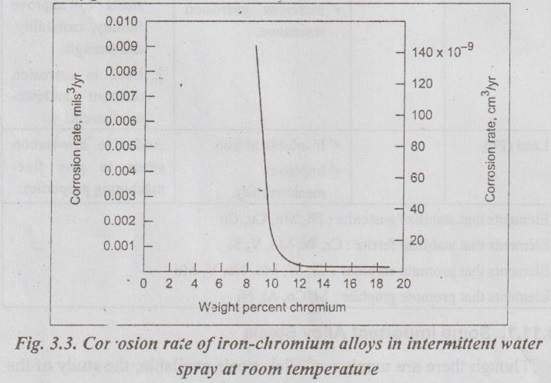
✓ The effect of chromium on corrosion of steel in water is shown in Fig.3.3. It can be noted from the Fig.3.3 that the higher the chromium content the more corrosion resistant is the steel.
✓ To enhance corrosion resistance, the stainless steels often contain other elements such as Cu, Al, Si, Ni and Mn. However, chromium remains as a predominant element.
3. Types of Stainless Steels
The stainless steels are divided into three classes on the basis of the predominant phase constituent of the microstructure as :
1. Austenitic stainless steels,
2. Ferritic stainless steels, and
3. Martensitic stainless steels.
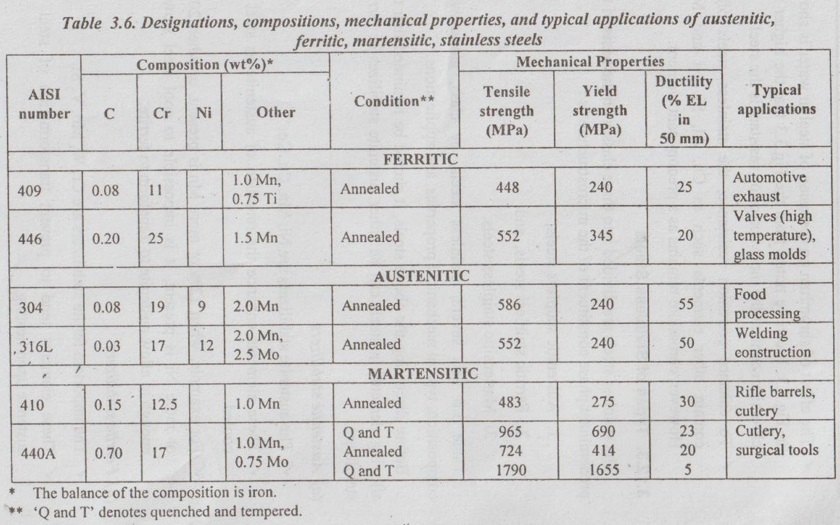
Table 3.6 lists several stainless steels, by class, along with composition, typical mechanical properties, and applications.
Before discussing the above steels, it should be reminded that the alloying elements in steels can be either austenite stabilizes or ferrite stabilizers.
(a) Austenite stabilizers
✓ The austenite stabilizers are Ni, Mn, Cu, Co.
✓ These elements enhance the retention of austenite as steel is cooled.
✓ For example, when 12% or more Mn is present, or when 20% or more Ni is present, it is impossible to cool steel slowly enough to allow austenite to transform to ferrite.
(b) Ferrite stabilizers
✓ The important ferrite stabilizers are Cr, W, Mo, V, Si.
✓ These elements tend to prevent transformation of steel to austenite upon heating.
Thus, whether a steel is austenitic, ferritic, or martensitic depends upon the balance between the amounts of austenite and ferrite stabilizers present, and the heating-cooling cycle to which the steel has been subjected. This can be explained in the following sections.
1. Austenitic Stainless Steels
✓ The austenitic stainless steels have the austenite structure retained at room temperature.
✓ It should be noted that when a layman speaks of stainless steel, it is usually implies an austenitic stainless steel.
✓ These steels are produced and used in greatest tonnage.
✓ Austenitic steels contain both chromium and nickel. When nickel is present, the tendency of nickel to lower the critical temperatures override the opposite effect of chromium. Thus the structure may become completely austenitic.
✓ In these steels, carbon contents are kept below 0.15% in order to minimize the formation of chromium carbides in the structure, as this would cause a reduction in corrosion resistance.
✓ Carbides may form in these steels if they are allowed to cool slowly from high temperature, or if they are heated in the range 500-700°C. The later condition may apply in the heat-affected zones adjacent to welds. The type of corrosion failure that can occur, due to the presence of carbide particles, is known as weld decay.
✓ In order to prevent the weld decay, the stabilizer such as titanium or niobium is added in small amounts with the baustenitic stainless steels.
✓ An austenitic stainless steel with improved strength, hardness and wear resistance can be achieved by introducing a fine dispersion of titanium nitride particles throughout the material (using the powder metallurgy methods).
✓ Composition: Typical composition of austenitic stainless steel time is given below:
C - 0.03 to 0.15%
Mn - 2 to 10%
Si - 1 to 2%
Cr - 16 to 26%
Ni 3.5 to 22%
P and S – Normal
Mo and Ti in some cases
✓ Properties: Some important properties of austenitic stainless steels are:
(i) Highest corrosion resistance.
(ii) Good strength and scale resistance at high temperature.
(iii) Non-magnetic.
(iv) Good ductility at cryogenic temperature i.e., below 0°C.
(v) Very tough and can be welded, forged or rolled.
✓ Applications: The typical applications of austenitic stainless steels include aircraft industry (engine parts), chemical processing (heat exchanger), food processing (kettles, tanks), household (cooking utensils), dairy industry (milk cans), transportation industry (trailers and railway cars), etc.
2. Ferritic Stainless Steels
✓ Ferritic stainless steels are ferritic in structure at all tempe- ratures upto their melting points. blow
✓ Ferritic stainless steels contain between 12 and 25% of chromium and less than 0.1% of carbon. doin to
✓ As austenite cannot be formed, it is impossible to form hard martensitic structures by quenching the steels from high temperature.
✓ This type of steel cannot be heat treated, but may be strengthened by work hardening.
✓ Composition: A typical composition of ferritic stainless steel is given below:
C - 0.08 to 0.10%
Mn - 1 to 1.5%
Si - 1%
Cr - 12 to 25%
✓ Properties: The important characteristics of the ferritic stainless steel are:
(i) They are magnetic.
(ii) They have good ductility.
(iii) They have great strength, toughness, and good resistance to corrosion.
(iv) These steels can be welded, forged, rolled, and machined.
✓ Applications: The typical applications of ferritic stainless steels include lining for petroleum industry, heating elements for furnaces, interior decorative work, screws and fittings, oil burner parts, etc.
3. Martensitic Stainless Steels
✓ Martensitic stainless steels contain between 12 and 25% of chromium, together with carbon contents ranging from 0.1 to 1.5%.
✓ The presence of carbon restores the a to y transition. These compositions can be heated to the austenitic range of temperatures and will transform to martensite upon cooling at suitable rates.
✓ Composition: A typical composition of martensitic stainless steel is given below:
C - 0.1 to 1.5%
Mn - 1%
Si - 1%
Cr - 12 to 25%
✓ Properties: Some of the important characteristics of martensitic stainless steels are:
(i) Good hardness, ductility, and thermal conductivity.
(ii) Good toughness and corrosion resistance.
✓ Applications: The typical applications of martensitic stainless steels include pumps and valve parts, rules and tapes, turbine buckets, surgical instruments, etc.
Note
For corrosion resistance and for a high degree of heat resistance, austenitic stainless steels (used in welded assemblies) are preferred to ferritic or martensitic stainless steels.
No comments:
Post a Comment