We have already discussed that the polymerisation is the process of forming a polymer by linking together of monomers.
POLYMERISATION
✔ We have already discussed that the polymerisation is the process of forming a polymer by linking together of monomers.
✔ The polymerisation mechanism can be divided into two categories as :
1. Addition polymerisation, and
2. Condensation polymerisation.
1. Addition Polymerisation
✓ Addition polymerisation, also known as chain reaction polymerisation, is a process by which two or more chemically similar monomers are polymerised to form long chain molecules.
✓ Polymerisation takes place only in unsaturated organic compounds. In this process, their double covalent bonds are broken and single bonds are formed in its place.
✓ Addition polymerisation yields a product that is an exact multiple of the original monomeric molecule.
✓ Addition polymerisation is usually instigated by the application of heat, light, pressure or a catalyst, as shown in Fig.4.7.
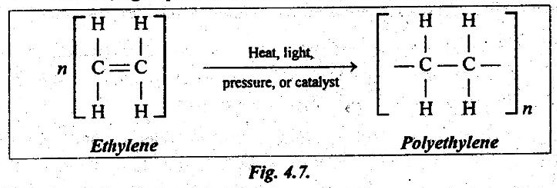
✓ It should be noted that in addition polymerisation process there is no loss of any substance.
1. Three Stages in Addition Polymerisation
The addition polymerisation occurs in three stages :
1. Initiation,
2. Propagation, and
3. Termination.
These three stages are demonstrated for polyethylene formation from ethylene monomer.
Stage 1: Initiation of addition polymerisation
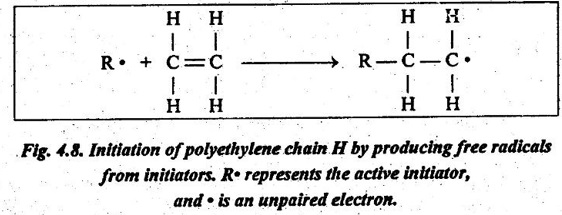
✔ During the initiation stage, an initiator (such as benzoyl peroxide) is added to the molecule.
✔ The initiator forms free radicals with a reactive site that attracts one of the caroon atoms of an ethylene monomer, as shown in Fig.4.8.
Stage 2: Propagation of the addition chain
✔ Once the chain is initiated, repeat units are added onto each chain at a high rate, as shown in Fig.4.9.
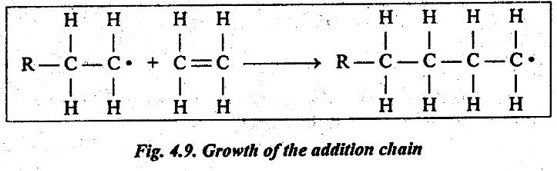
✔ The chain growth is relatively quick. For example, the time required to grow a molecule consisting of 1000 mer units is on the order of 10-2 to 10-3 s.
Stage 3: Termination of addition polymerisation
✔ The chains may be terminated by two mechanisms. They are :
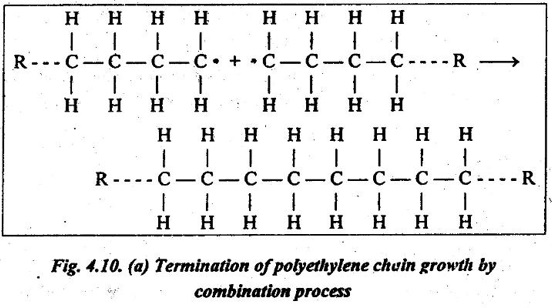
1. The active ends of two propagating chains may react or link together to form a non-reactive molecules, as shown in Fig.4.10 (a). This process is called combination. This terminates the growth of each chain.
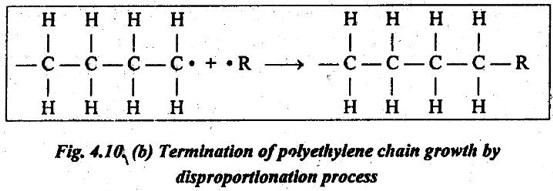
2. The active end of one chain may remove a hydrogen atom from a second chain by a process known as disproportionation. This reaction terminates two chains, as shown in Fig.4.10 (b).
2. Applications
Addition polymerisation is used in the synthesis of polyethylene, polypropylene, polyvinyl chloride, and polystyrene, and many copolymers.
Note
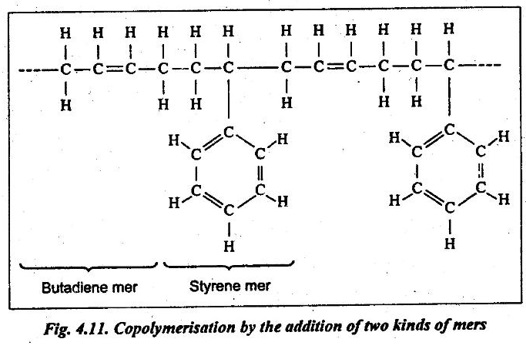
✔ Another kind of addition polymerisation is copolymerisation. Copolymerisation is the addition of two or more different monomers.
✔ A copolymer may have properties quite different from those of either component member. Thus a wide variety of plastics may be obtained by this process.
✔ Example: Styrene and butadiene combines to give a copolymer of styrene and butadiene, as shown in Fig.4.11.
2. Condensation Polymerisation
✔ Condensation polymerisation, also known as step-growth polymerisation, is the formation of polymers by stepwise intermolecular chemical reactions that normally involve at least two different monomers.
✔ In contrast to addition polymerisations, the condensation polymerisations form a small molecular weight by-product such as water or CH3OH in addition to the polymer final product.
✔ In this, the reactant products will not have the chemical formula of the mer repeat unit, and the intermolecular reaction occurs every time a mer repeat unit is formed.
1. Illustrations
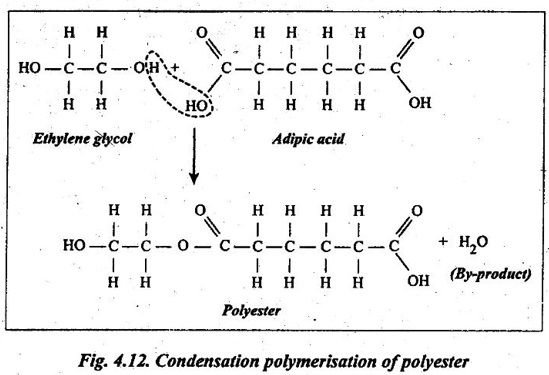
1. Formation of a polyester from the reaction between ethylene glycol and adipic acid is illustrated in Fig.4.12.
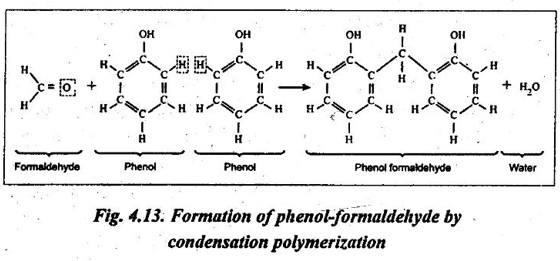
2. The condensation polymerisation of Bakelite i.e., phenol formaldehyde is illustrated in Fig.4.13.
2. Characteristics of Condensation Polymerisation
✔ The reaction times for condensation are generally larger than for addition polymerisation.
✔ Like addition polymerisation, various chain length can be produced, yielding a molecular weight distribution.
✔ Condensation reactions often produce trifunctional monomers capable of forming cross-linked and network polymers.
3. Applications
The condensation polymerisation is used in the synthesis of thermosetting polyesters and phenol formaldehyde (Bakelite), the nylons, and the polycarbonates.
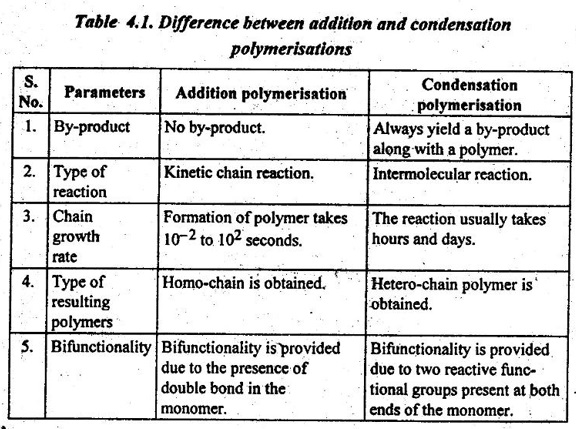
3. Addition Polymerisation Vs Condensation Polymerisation
Table 4.1 presents the difference between addition and conden- sation polymerisation processes.
No comments:
Post a Comment