Engineering ceramics, are also known as technical/industrial ceramics or advanced ceramics, are those ceramics that are specially used for engineering applications or in industries.
ENGINEERING CERAMICS
1. What are Engineering Ceramics ?
✓ Engineering ceramics, are also known as technical/industrial ceramics or advanced ceramics, are those ceramics that are specially used for engineering applications or in industries.
✔ Engineering ceramics are mainly oxides, carbides, sulphides, and nitrides of metals.
2. Characteristics of Engineering Ceramics
Any ceramic can be said to be an engineering ceramic if it can satisfy one or more of the following requirements:
(a) High resistance to abrasion and wear.
(b) High strength at high temperature.
(c) Good chemical stability.
(d) Good electrical insulation characteristics.
3. Classification of Engineering Ceramics
Engineering ceramics↑ can be classified into oxides, carbides, sulphides, nitrides, metalloids, or intermetallics,↑↑ as shown in Table 4.7. The Table 4.7 also presents examples for each class and their typical applications.
† How ceramics are made? Many ceramic components are made by the process of pressing a powder into a compact, followed by sintering. Other ceramic components are made by gluing the powders together with glasses, which is called as vitrification.
†† Intermetallic compounds are compounds that are formed by the combination of two metals. They are not alloys, but sometimes they have metallic properties and sometime they have properties that are like those of ceramic materials. For more details, refer Unit 1, Section 1.3.1.

Now we shall discuss the properties and applications of the following important engineering ceramics, in the following sections:
1. Alumina (Al2O3),
2. Silicon carbide (SiC),
3. Silicon nitride (Si3N4),
4. Partially stabilised zirconia (PSZ), and
5. Sialons.
1. Alumina (Al2O3)
✔ Alumina is nothing but an aluminium oxide (Al2O3), which is the oldest engineering ceramic.
✔ Alumina is produced from bauxite (Al2O3 · 2 H2O), which is the main ore from which metallic aluminium is manufactured.
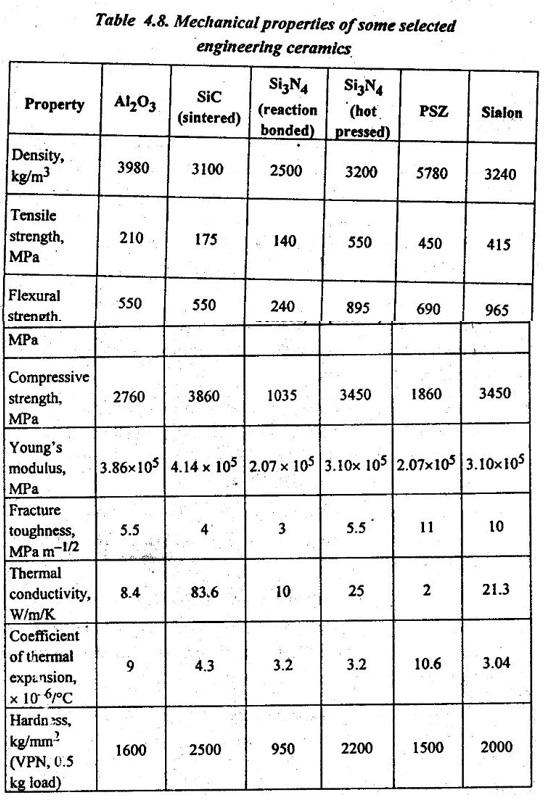
✔ Characteristics of alumina: Typical mechanical and physical properties of alumina are shown in Table 4.8. Some notable properties are the following:
1. Aluminas have excellent hardness, wear resistance and chemical inertness properties.
2. They are more stiffer than steels.
3. They are more stronger in compression than many hardened tool steels.
4. They retain 50% of their room temperature strength at elevated temperature (about 1093°C).
5. They possess very good environmental resistance.
6. They are susceptible to overheating because they are poor thermal conductors.
7. They possess low neutron absorption cross-section. This property enables them to find application in nuclear equipment.
✔ In practice, high alumina ceramics (i.e., containing 75 - 99.8% Al2O3) are extensively used.
✔ Alumina is blended with other ceramics such as zirconia to improve its tensile and toughness properties.
✔ Applications of aluminas :
1. High alumina ceramics are used for the manufacture of spark- plug insulators, ceramic/metal assemblies in vacuum tubes, substrates for the deposition of electronic microcircuits, and metal-cutting tool tips.
2. They are suitable for any type of load-bearing application. They are used for rocket nozzles, pump impellers, pump liners, check valves, nozzles subject to erosion, and for support members in electrical and electronic devices.
3. Some unique applications of alumina are in dental and medical use, including restoration of teeth, bone filler, and orthopaedic implants.
4. These materials find application in nuclear equipment.
5. Much of the alumina produced is used in military armour plating. These armour plates protect against missiles fired from high-powered rifles.
† Chromium-doper alumina is a laser.
2. Silicon Carbide (SiC)
✔ Silicon carbide (SiC) is a hard, semiconducting ceramic material. In fact, it is the hardest of the traditional abrasive materials.
✔ Silicon carbides have been used as abrasives for grinding wheels and for bonded abrasive papers for many years.
✔ Types of silicon carbide: The two principal types of silicon carbide are :
1. α-SiC: The α silicon carbide is made by the reduction of silica sand with carbon in an arc furnace. It has a hexagonal crystalline structure.
2. β-SIC: The B-silicon carbide is produced by a vapour phase reactions. It has a cubic crystalline structure.
✔ Types of silicon carbide ceramics: Depending upon the mode of manufacture, the following different types of silicon carbide ceramics are available:
1. Reaction bonded silicon carbide.
2. Clay-bonded silicon carbide.
3. Hot-pressed silicon carbide.
4. Sintered silicon carbide.
5. Recrystallised silicon carbide.
6. Nitride-bonded silicon carbide.
✔ Characteristics of silicon carbides: Some of the important properties of SiC are given below (see also Table 4.8):
1. Silicon carbides have higher tensile strength, stiffness, hardness, and lower density than aluminium oxides.
2. They provide outstanding oxidation resistance at tempera- tures even above the melting point of steel (upto 1500°C).
3. They possess the highest thermal conductivity when com- pared with most engineering ceramics.
4. They have better dimensional stability and polishability.
5. They are abrasion resistant and wear resistant.
6. They are also chemical resistant.
7. They are not very tough.
8. They are expensive with limited availability of shapes and sizes.
✔ Applications of silicon carbides :
1. Silicon carbides are widely used as abrasives for grinding wheels and for bonded abrasive papers.
2. They are used for precision optical mirrors and special fixtures for the semiconductor industry.
3. They are also used as coatings (i.e., cladding material) for metals, composites, and other ceramics to provide protection at very high temperatures. Example, they are used for nuclear-reactor fuel elements, mechanical seals, bearings, and engine components.
4. They are used for refractory tubes and containers.
5. They are also formed as fibres and whiskers for use as reinforcement in composite materials.
3. Silicon Nitride (Si3N4)
✔ Silicon nitride (Si3N4) is a very useful engineering ceramics which is fully resistant to most strong acids and to molten aluminium and other low melting point metals.
✔ Types of silicon nitride ceramics: Depending upon the mode of manufacture, there are different types of silicon nitride ceramics available. The four important silicon nitride ceramics are:
1. Reaction bonded silicon nitride (RBSN),
2. Hot pressed silicon nitride (HPSN),
3. Sintered silicon nitride (SSN), and
4. Pressureless sintered silicon nitride (PSSN).
✔ Characteristics of silicon nitrides: Some of the important properties of silicon nitrides are given below (see also Table 4.8):
1. Silicon nitrides are fully resistant to most strong acids, to molten aluminium, and to other low melting point metals.
2. They exhibit greater thermal shock resistance than many other ceramics.
3. They have lower density and hence the lower weight than most other engineering ceramics.
4. They have low thermal expansion.
5. They exhibit better toughness than Al2O3 and SiC.
6. They are 50% more stiffer than steels.
7. They are very porous, which lowers strength and mechanical properties.
✔ Applications of silicon nitrides :
1. Silicon nitrides are widely used as cutting tool materials.
2. They are used for automotive and gas turbine parts; for balls, rollers, and racers for bearings; for diesel engines, hot extrusion pipes, and pump parts.
3. They are also used as pouring tubes for aluminium, and for high-temperature engineering components.
4. Partially Stabilised Zirconia (PSZ)
✔ Partially stabilised zirconia (PSZ) is nothing but a zirconium oxide (ZrO2), that has been blended and sintered with some other oxide such as magnesium oxide (MgO), calcium oxide (CaO), and yttria (Y2O3), to control crystal structure transformations.
✔ Zirconia (ZrO2) has been used for many years as a high- temperature crucible and furnace refractory where temperatures upto 2500°C must be sustained.
✔ Why zirconia should be stabilised ?
The oxide of zirconium (ZrO2) exists in three different crystalline modifications. The ZrO2 has a cubic structure at elevated tempera- tures, during cooling, this transforms first into a tetragonal structure. Then at room temperature, it transforms into a monoclinic crystal structure. On cooling, transformation (from cubic structure to monoclinic structure) causes cracking because of a 3% volume change. So it is difficult to fabricate pure ZrO2 ceramics. In order to avoid cracking, zirconia is stabilised by adding a 'stabilisng' oxide such as MgO, CaO, or Y2O3.
✔ A fully stabilized zirconia (FSZ) contains 18% of any of the stabilising oxides, but partially stabilized zirconia (PSZ) con- tains about 5% of a stabilizing oxide.
✔ Characteristics of PSZ: Some of the important properties of PSZS are given below (see also Table 4.8):
1. Partially stabilised zirconias have better fracture toughness than the other high-performance ceramics.
2. They have better tensile strength than that of alumina and some of the other engineering ceramics.
3. They possess low thermal conductivity, which make them as good thermal insulators.
4. They have thermal expansion and modulus of elasticity similar to that of steels.
✔ Applications of PSZs :
1. PSZs are used for superalloy rotor blades in jet turbines.
2. Since PSZs ́are ‘environmentally friendly' inside the human body, they are finding use in implantology. They are used for the manufacture of artificial hip joints.
3. Nowadays PSZs replace metals in internal combustion engines.
5. Sialons (Si3Al3O3N5)
✔ The name sialon is an acronym derived from the ingredients involved, namely Si – Al – O - N.
✔ That is, the sialons are derivatives of silicon nitride. Sialons are formed when aluminium and oxygen partially substitute for silicon and nitrogen in silicon nitride.
✔ The general form of the material is Si6-AI2O2N8-z. When z = 3, the formula obtained is Si3Al3O3N5, which is termed as sialon.
✔ Characteristics of sialons: Some of the important properties of sialons are given below (see also Table 4.8):
1. Sialons retain their hardness at higher temperatures than does alumina.
2. They are tough and have higher strength.
3. They possess good mechanical properties.
4. They are relatively light-weight materials with low coefficient of thermal expansion.
✔ Applications of sialons :
1. Sialons are used for cutting tool materials, dies for drawing wire and tubes, rock-cutting and coal-cutting equipment, nozzles and welding shields.
2. They are used for the manufacture of thermocouple sheaths, radiant heater tubes, impellers, small crucibles and other purposes involving temperatures upto 1250°C.
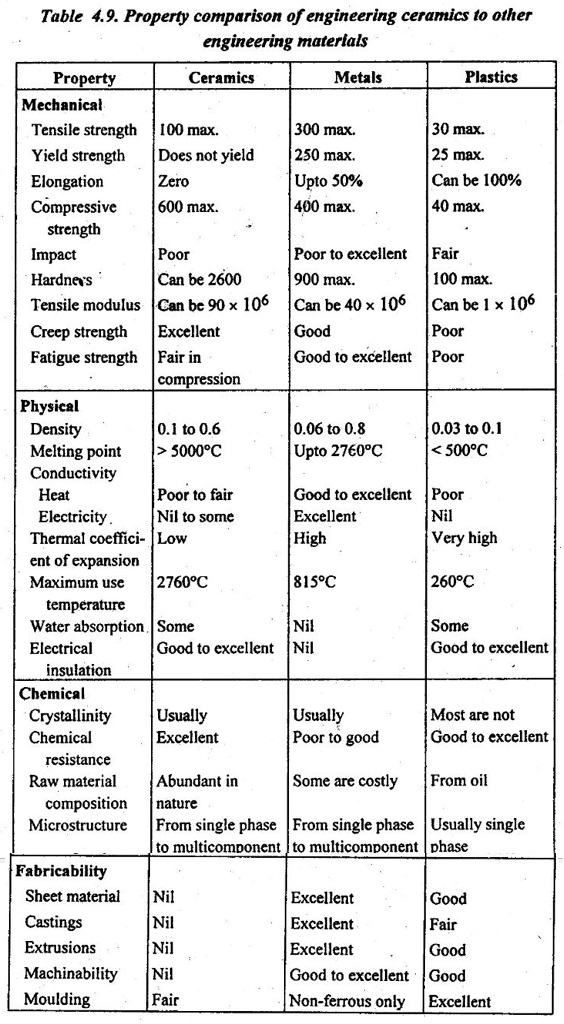
4. Comparison of Engineering Ceramics to Other Engineering Materials
Table 4.9 presents the property comparison of engineering ceramics with metals and plastics. This will enable us to better understand the properties, the reasons for their selection, and the suitability of the engineering ceramics, metals, and plastic materials.
No comments:
Post a Comment