As we have discussed, the ferrous materials are those materials which contain-iron as their prime constituent.
FERROUS MATERIALS
1. Introduction
✓ As we have discussed, the ferrous materials are those materials which contain-iron as their prime constituent.
✓ More than 90% by weight of the metallic materials used by human bein 's are ferrous materials.
✓ The ferrous metals are extensively used in engineering due to the following three factors:
1. Iron-based components are relatively abundant and are widely distributed throughout the world.
2. Ferrous materials can be produced very economically.
3. Ferrous materials are versatile. Therefore wide range of mechanical and physical properties of ferrous materials can be achieved.
Thus the availability, comparatively low cost, and the wide range of properties that can be achieved have made ferrous hot materials of great importance.
✓ The principal disadvantage of many ferrous alloys is their susceptibility to corrosion.
† The term ferrous materials represents both ferrous metals and ferrous alloys. It should be noted that pure metals are rarely used in engineering application because these are very soft and ductile. That's why for all practical purposes mostly alloys are used. An alloy is a homogeneous mixture of two or more metals or a metal and a non-metal.
The same is true with making gold ornaments. Pure gold is rarely used; always impurities are added to enhance the required mechanical properties.
2. Classification of Ferrous Materials
A taxonomic classification scheme for the various ferrous materials is presented in Fig.3.2.
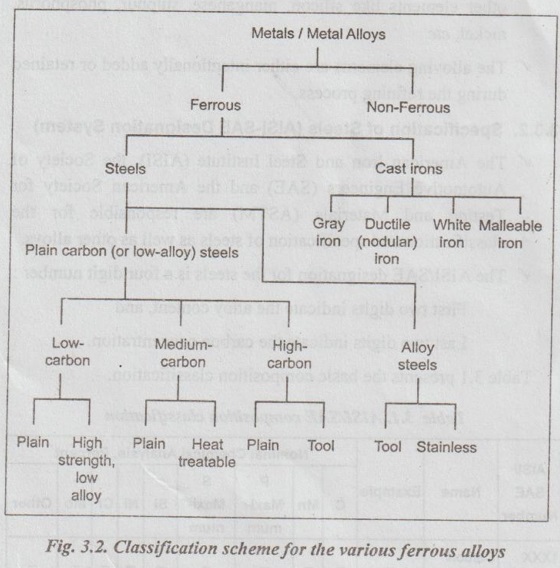
As shown in Fig.3.2, ferrous materials fall into two broad categories: steel and cast iron-based on the amount of carbon in the alloy composition. Steel generally contains between 0.05 and 2.0% weight carbon. The cast irons generally contain between 2.0 and 4.5% weight carbon.
3. STEELS
1. What are steels?
✓ Steels are alloys of iron and carbon. However, steels contain other elements like silicon, manganese, sulphur, phosphorus, nickel, etc.
✓ The alloying elements are either intentionally added or retained during the refining process.
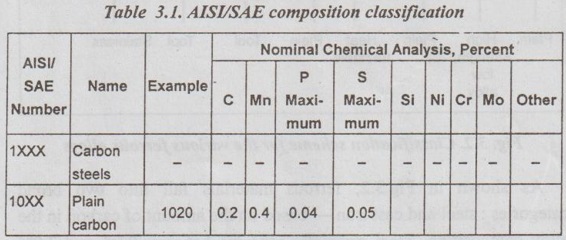
2. Specification of Steels (AISI-SAE Designation System)
✓ The American Iron and Steel Institute (AISI), the Society of Automotive Engineers (SAE) and the American Society for Testing and Materials (ASTM) are responsible for the classification and specification of steels as well as other alloys.
✓ The AISI/SAE designation for the steels is a four digit number:
First two digits indicate the alloy content, and
Last two digits indicate the carbon concentration.
Table 3.1 presents the basic composition classification.
† How steels are produced?
Steels are typically produced in two ways:
1. By refining iron ore in blast furnace, or
2. By recycling scrap steel in an arc furnace.
However, the study of steel production is beyond our scope.
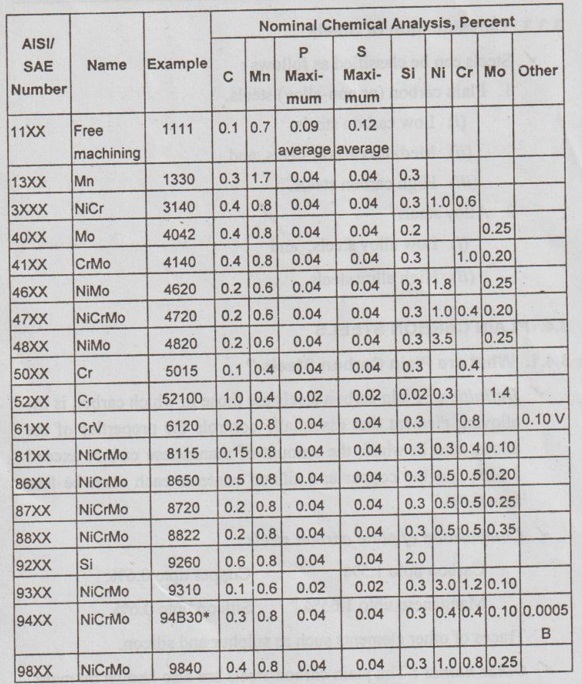
* 'B' refers to the presence of boron
✓ Illustration: From Table 3.1, it can be seen that for plain carbon steels, the first two digits are 1 and 0; alloy steels are designated by other initial two-digit combinations (e.g., 13, 41, 43). The third and fourth digits represent the weight percent carbon multiplied by 100. For example, a 1080 steel is a plain carbon steel containing 0.80% C; similarly a 4340 steel is a Mo-Cr-Ni alloy with 0.40% C.
3. Classification of Steels
✓ Steels can be classified as follows:
1. Plain carbon (or non-alloy) steels.
(i) Low carbon steels.
(ii) Medium carbon steels, and
(iii) High carbon steels.
2. Alloy steels
(i) Low alloy steels, and
(ii) High alloy steels.
4. PLAIN CARBON STEELS
1. What are Plain Carbon Steels?
✓ Definition: Plain carbon steels are those in which carbon is the alloying element that essentially controls the properties of the alloys, and in which the amount of manganese cannot exceed 1.65% and the copper and silicon contents each must be less than 0.6%.
✓ Composition of plain carbon steels:
Carbon upto 1.5%
Copper upto 0.6%.
Manganese upto 1.65%
Silicon upto 0.6%
Traces of other elements such as sulphur and silicon.
✓ Other names: The plain carbon steels are also known by many terms such as carbon steels, non-alloy steels, and straight- misla carbon steels.
2. Characteristics of Plain Carbon Steels
The important properties of plain carbon steels are given below:
1. Plain carbon steels are the moderately priced steels (due to the absence of large amounts of alloying elements).
2. They are sufficiently ductile to readily formed.
3. Plain carbon steels are available in almost all product forms: Sheet, strip, bar, plates, tube, pipe, wire.
3. Applications of Plain Carbon Steels
✓ Plain carbon steels are used for mass-production products such as automobiles and applicance.
✓ They also find applications in the production of ball bearings, base plates, housings, chutes, structural members, etc.
4. Classification of Plain Carbon Steels
The plain carbon steels can be subdivided into:
1. Low-carbon steels: Those contain less than 0.25% carbon.
2. Medium-carbon steels: Those containing between 0.25 and 0.60% carbon.
3. High-carbon steels: Those containing more than 0.60% carbon.
5. LOW CARBON STEELS
1. What are Low-Carbon Steels?
✓ The low-carbon steels represent the largest tonnage of all the steels produced.
✓ The low-carbon steels are those steels that contain less than about 0.25% carbon.
✓ The low-carbon steels are also known as mild steels.
2. Characteristics of Low-Carbon Steels
The important properties of low-carbon steels are given below:
1. Low-carbon steels are relatively soft and weak.
2. They cannot be hardened appreciably by heat treatment.
3. They possess good formability and weldability.
4. Strengthening of low-carbon steels are accomplished by cold work.
5. They have outstanding ductility and toughness.
6. The microstructure of low-carbon steels consist of ferrite and pearlite constituents.
7. Of all steels, the low-carbon steels are the least expensive to produce.
3. Applications of Low-Carbon Steels
Typical applications of low-carbon steels include automobile body components, structural shapes (I-beams, channel, and angle iron), and sheets that are used in pipelines, buildings, bridges, and tin cans.
No comments:
Post a Comment