A plastic may be defined as an organic polymer, which can be moulded into any desired shape and size with the help of heat, pressure, or both.
PLASTICS
1. What are Plastics?
fatty acid amides, fluoro- dispersions, glycerides, petrolatum, etc.
✔ A plastic may be defined as an organic polymer, which can be moulded into any desired shape and size with the help of heat, pressure, or both.
✔ Nowadays plastics are extensively used in engineering appli- cations due to their important properties such as low price, colour range, toughness, water resistance, low electrical and thermal conductivity, ease of fabrication, etc.
✔ Standard forms in which plastics are available include powders, sheets, films, rods, tubes, and liquids.
Missing 4.18
✔ Thermoplasts are relatively soft and ductile.
✔ Since thermoplasts have low melting temperatures and can be repeatedly moulded and remoulded to the desired shape, they have a good resale/scrap value.
Mechanism: It may be noted that most of the thermoplastics are formed by addition polymerisation. We know that the addition polymerisation produces only linear polymers i.e., chain molecules or linear molecules. Therefore thermoplastics can be mechanically deformed and softened at high temperature. Also they can be easily moulded due to the absence of cross links. On cooling, they are hardened and they regain their original low temperature properties.
Examples: Some of the important thermoplastics are :
1. Polythenes,
2. Polystyrenes,
3. Polyvinyls,
4. Acrylics,
5. Polyamides (Nylones),
6. Polytetrafluoro ethylenes (Teflon), and
7. Cellulosics.
2. Thermosetting Plastics
✓ Thermosetting plastics, also known as thermosets, are plastics which become permanently hard when heat is applied and do not soften upon subsequent heating.
✓ That is, thermosetting plastics once set cannot be soften on heating. Thus they cannot be remoulded/reshaped again and again. That's why they do not have a resale/scrap value.
✓ The thermosetting plastics are generally stronger, harder, more brittle, more resistant to heat and solvents than thermoplastics.
Mechanism: It may be noted that most of the thermosetting plastics are formed by condensation polymerisation. We know that the condensation polymerisation produces cross-linked molecules. Cross linked molecules are composed of long molecules linked to each other in three dimensions by primary or valence bonds. They are not broken by heat until the compound is decomposed. Once the product is heated to an excessive temperature, where cross-links are broken, an irreversible decomposition takes place. Moreover, due to these cross- linked molecules the thermosetting plastics cannot be softened once they are moulded, even at high temperatures.
Examples: Some of the important thermosetting plastics are:
1. Pólyesters,
2. Phenolic,
3. Urea formaldehyde,
4. Melamine formaldehyde, and
5. Epoxides.
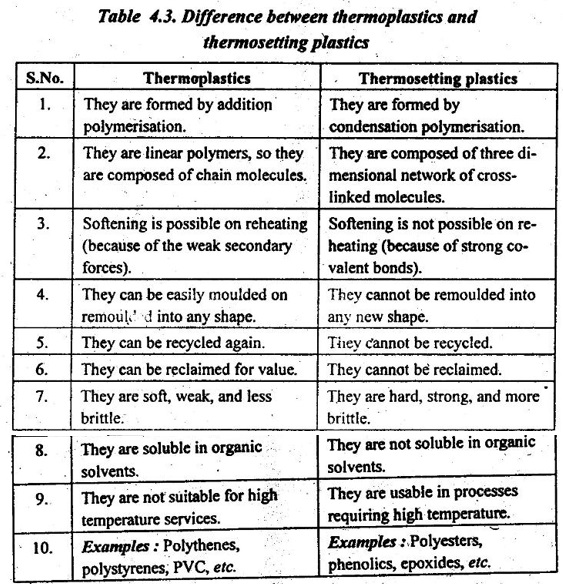
5. Thermoplastics Vs Thermosetting Plastics
Table 4.3 presents the difference between thermoplastics and thermosetting plastics.
No comments:
Post a Comment