As stated earlier, cast irons can be defined as the ferrous alloys en with greater than 2% carbon.
CAST IRONS
1. Introduction
✓ As stated earlier, cast irons can be defined as the ferrous alloys en with greater than 2% carbon. They also contain small amounts of silicon, sulphur, manganese, and phosphorus.
✓ In principle, cast irons are eutectic alloys of iron and carbon.
✓ Cast irons are the least expensive of all metals. This is because iron is the second most plentiful metallic resource next to aluminium.
✓ As the name implies, molten cast iron can be cast into any complex shapes and can be easily machined to required tolerances.
2. What are the Features that make Cast Iron an Important Material?
The following features make cast iron an important material.
1. It is a cheap metallurgical substance.
2. Good mechanical rigidity and good strength under compression.
3. Easy castability.
4. Good machinability can be achieved when a suitable composition is selected.
5. High-duty cast irons can be produced by further treatment of irons of suitable composition. For example, spheroidal- graphite irons are strong, whilst malleable irons are tough.
3. Composition of Cast Iron
The composition of ordinary cast irons is given below:
Carbon 3.0 - 4.0%
Sulphur upto 0.1%
Silicon 1.0 - 3.0%
Phosphorus upto 1.0%
Manganese 0.5 -1.0%
4. Effect of Composition Elements on Cast Iron
The effect of the above said impurities i.e., composition elements on cast iron are given below:
1. Carbon
✓ Carbon may be present in the structure either as flakes of graphite or as a network of hard, brittle iron carbide i.e., cementite. uqlue to
✓ If a cast iron contains more of the brittle cementite, then its mechanical properties will be poor. That's why for most engineering purposes, it is desirable to have carbon in the form of small flakes of graphite.
✓ Cementite is a silvery-white compound. If an iron containing much cementite is broken, the fractured surface will be silvery white. This is because the piece of iron breaks along the brittle benin cementite networks. The iron so obtained is known as a white iron.
✓ In contrast, if an iron contains much graphite, its fractured surface will be grey, due to the presence of graphite flakes in the structure. Such an iron is termed as a grey iron.
2. Silicon
✓ Silicon to some extent governs the form in which carbon is present in cast iron. so
✓ It causes the cementite to be unstable, so that it decomposes, thus releasing free graphite.
✓ Therefore a high-silicon iron tends to be a grey iron, whilst a low-silicon iron tends to be a white iron.
3. Sulphur
✓ Sulphur tends to stabilize cementite. Thus it helps to produce a white iron.
✓ However it should be noted that sulphur causes excessive brittleness in cast iron. Therefore always it should be kept to the minimum amount.
4. Manganese
✓ Manganese toughens and strengthens an iron.
✓ It helps to exert a controlling influence over the harmful effect of sulphur.
5. Phosphorus
✓ Phosphorus aids fusibility and fluidity in cast iron.
✓ However, like silicon, it induces brittleness. Therefore, it should be kept to a minimum amount.
5. The Influence of Cooling Rate on the Properties of a Cast Iron
✓ Slow cooling rates during solidification of castings (as obtained in sand moulds) generally allow for graphite formation in iron resulting in grey irons.
✓ More rapid solidification will tend to give white iron structures.
The effect of silicon and the cooling rate on the cast iron can be simply represented as below:
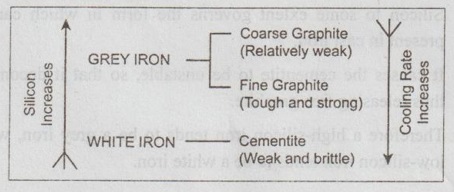
From the above illustration, it may be noted that the engineers must strike a balance between the silicon content of the iron and the cooling rate while producing the required cast iron.
Thus for the manufacture of castings of thin sections, the cast irons must have a higher silicon content and slow cooling rate than that used for the casting of heavier sections.
6. The Effect of Heat Treatment on Cast Irons
✓ The prolonged heating of a white iron cause graphitisation to occur. This phenomenon is used as the basis for the production of malleable irons.
✓ Graphite is less dense than cementite. The cementite decomposes into ferrite and graphite during heating (at emperature about 700°C). The decomposed ferrite and graphite will occupy more space than did the original cementite structure. This phenomenon is termed as the growth of cast irons.
✓ The 'growth' of cast irons leads to warping of the casting, and the formation of cracks at the surface. This effect can be seen normally in high-temperature services.
✓ The prevention of 'growth' in cast irons can be achieved by using a high-silicon-content iron, in which cementite is fully graphitised state before being put into use.
7. Types of Cast Irons
There are five major types of cast irons, as shown in Fig.3.4
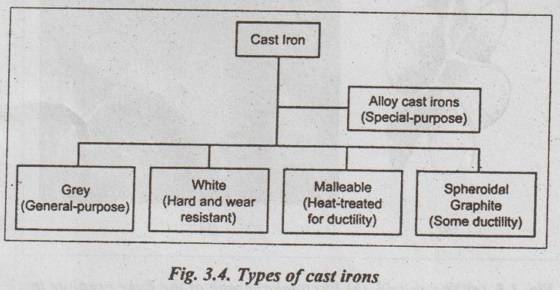
Now, we shall discuss the above cast irons, one by one, in the following sections.
No comments:
Post a Comment