Almost all biological systems are built of polymers which not only perform mechanical functions (like wood, bone, cartilage, leather) but also contain and regulate chemical reactions (leaf, veins, cells).
POLYMERS
1. Introduction
Almost all biological systems are built of polymers which not only perform mechanical functions (like wood, bone, cartilage, leather) but also contain and regulate chemical reactions (leaf, veins, cells). People have used these natural polymers for thousands of years now. Modern scientific researches have made possible the development of numerous polymers, which are synthesized from small organic molecules.
Many of our useful plastics, rubbers, and fibre materials are synthetic polymers. In fact, in some applications polymers replaced metallic materials. The use of synthetic polymers have increased largely during recent decades. This is due to their many useful properties, coupled with comparatively low cost.
2. Characteristics of Polymers
Some of the attractive characteristics of polymers are :
1. Low density.
2. Good thermal and electrical insulation properties.
3. High resistance to chemical attack.
4. Ease of fabrication.
5. Relatively low cost.
Some of the disadvantages of polymer materials are:
1. Low strength.
2. Low elastic modulus values.
3. Low softening temperatures.
However, the low strength and stiffness can be improved by fibre reinforcement of polymers.
3. What are Polymers?
✔ The term polymer* is derived from two Greek words 'poly' and 'mers'. The term 'poly' means 'many', and the term 'mer' means 'parts or units'. Thus polymers are composed of a large number of repeating units of small molecules called monomers.
✓ Monomers: Monomers are the small molecules which combine to form a polymer. They are also called repeating units as they combine repeatedly to form a polymer. The number of repeating units in a polymer is known as degree of polymerisation or D.P. value.
✓ Polymerisation is a reaction which involves the union of small molecules to form molecules having higher molecular weight called polymer.
✓ Therefore, a polymer is made up of thousands of monomers joined together to form a large molecule.
✓ Polymers may be defined as giant organic, chain-like molecules having molecular weight↑↑ from 10000 to more than 1,000,000 g.mol-1.
4. Classification of Polymers
The basic classification scheme of polymers is illustrated in Fig.4.1.
† In practice, the term polymer is used interchangeably with the term plastic.
†† Molecular weight: It is the sum of the atomic weights of all the atoms in a molecule. Molecule is a group of atoms that are bound together by primary interatomic bonds.
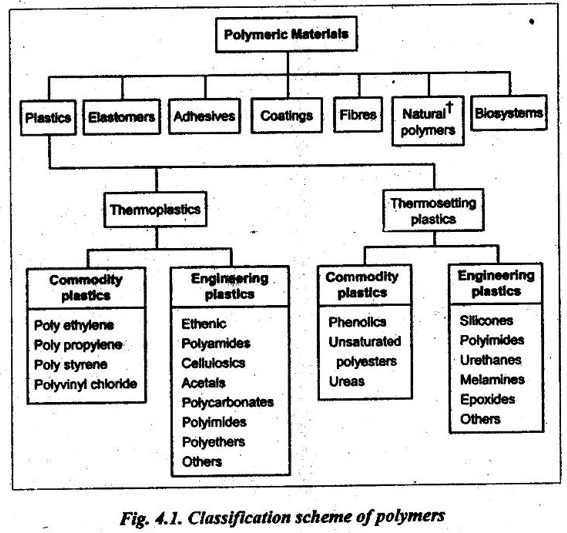
However, our study is limited only to thermoplastics and thermosetting plastics.
5. Terminology Used in Polymers
The important terms used in the study of polymers have been explained below :
1. Monomer: It is a small molecule consisting of a single mer i.e., a single unit/blocking block.
† Natural polymers include proteins, cellulose, resins, starch, shellac, and lignin. They are commonly found in leather, furr, wool, cotton, silk, furr rope, wood and many others. Other than natural polymers are generally called as synthetic polymers.
2. Polymer: It is a macromolecule formed by the repeated linking of many monomers.
3. Polymerisation : It is the process of forming a polymer.
4. Homopolymer: It is a polymer made out of identical monomer.
In other words, when all the repeating units along a chain are of the same type, the resulting polymer is called a homopolymer.
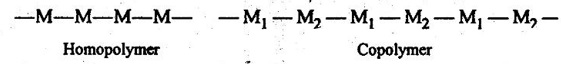
5. Copolymer: It is a polymer which is obtained by adding different types of monomers.
6. Degree of polymerization : It is the number of repetitive units (or mers) present in one molecule of a polymer. It is a parameter used to designate the average chain size of a polymer. Mathematically,
Degree of polymerization = Molecular weight of a polymer / Molecular weight of a single monomer
7. High-polymers: Solid polymers which have very high molecular weights (ranging between 10,000 and 1,000,000 g/mol) are called high-polymers.
8. Oligo-polymers: Oligo-polymers or oligomers are liquid/gas polymers with very short chains (having molecular weights on the order of 100 g/mol).
Types of Homopolymers
9. Linear polymers: Linear polymers are those in which the mer units are joined together end to end in single chains, as shown in Fig.4.2 (a). For linear polymers, there there may be extensive Van der Waals boundary between the chains.
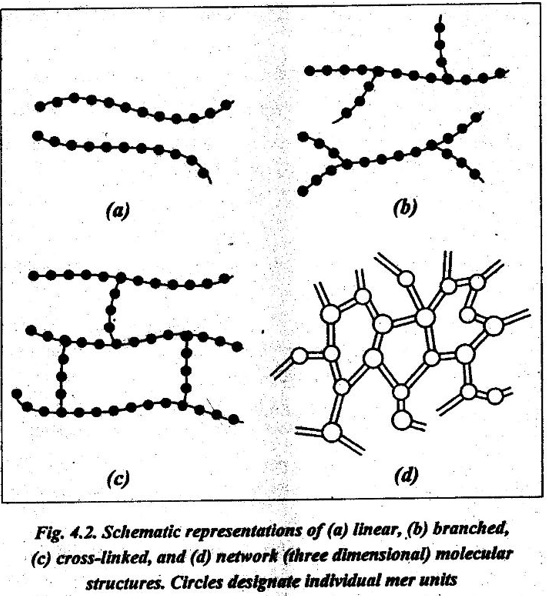
10. Branched polymers: Branched polymers are those in which side-branch chains are connected to the main ones, as shown in Fig.4.2 (b).
11. Cross-linked polymers: In cross-linked polymers, adjacent linear chains are joined one to another at various positions by covalent bonds, as shown in Fig.4.2 (c).
12. Network polymers: Network polymers have three active covalent bonds (known as trifur.ctional mer units), which form three- dimensional networks instead of the linear chain framework, as shown in Fig.4.2 (d).
Types of Copolymers
Consider a copolymer that is composed of two mer units as represented by ● and ● in Fig.4.3. Depending on the polymerisation process and the relative fractions of these mer types, we have different sequencing arrangements along the polymer chains. They are:
13. Random copolymer: In this, the two different units are randomly dispersed along the chain, as shown in Fig.4.3 (a).
14. Alternating copolymer: In this, the two mer units alternate chain positions, as shown in Fig.4.3 (b).
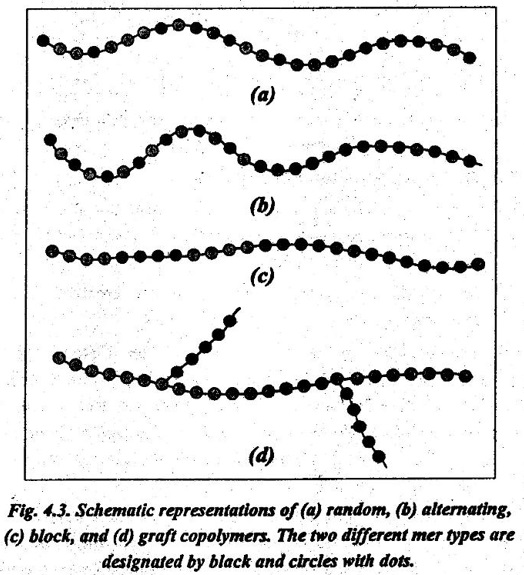
15. Block copolymer: In this, the identical mers are clustered in locks along the chain, as shown in Fig.4.3 (c).
16. Graft copolymer: In this, homopolymer side branches of one ype may be grafted to homopolymer main chains that are composed ›f a different mer, as shown in Fig.4.3 (d).
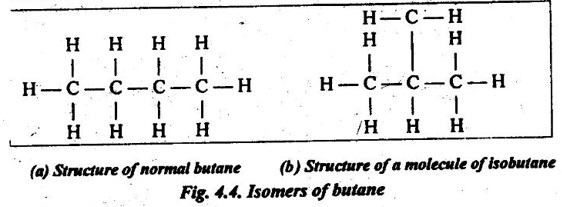
17. Isomerism: It is a phenomenon wherein different atomic configurations are possible for the same configuration. For example, here are two isomers for butane (C4H10) as shown in Fig.4.4.
6. Molecular Structure of Polymers
✔ As we know, most polymers are organic in origin; many organic materials are hydrocarbons. In other words, most polymers are hydrocarbons.
✔ In hydrocarbons, carbon and hydrogen combine in the relationship Cn H2n + 2, known as paraffins.
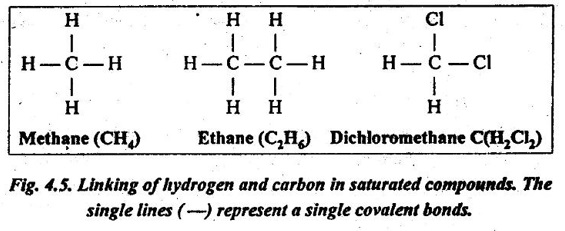
✔ Theoretically, the hydrocarbons can be linked together indefinitely to form very large molecules, as illustrated in Fig.4.5. The bonds between the atoms are single pairs of covalent electrons. Because there is no provision for additional atoms to be added to the chain, such molecules are said to be saturated.
In other words, a compound in which all the valence bonds of the carbon atoms are satisfied is said to be saturated. Such saturated molecules have strong intramolecular bonds, but the intermolecular bonds are much weaker.
✔ A bond between two carbon atoms may involve the sharing of two pairs of electrons. This is termed as a double bond. For example, in ethylene (C2H4), the two`carbon atoms are doubly bonded together, and each is also bonded to two hydrogen atoms, as illustrated in Fig.4.6 (a). In some cases, triple bonds also exist. For example, in acetylene (C2H2), there is a triple bond between two carbon atoms, as shown in Fig.4.6 (b).
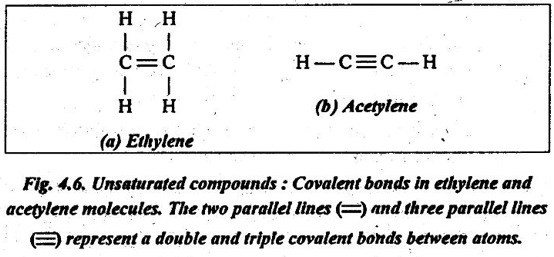
✔ In these double and triple covalent bonds molecules, each carbon atom is not bonded to the maximum other atoms. So these compounds are termed as unsaturated.
In other words, a compound in which the valence bonds of the carbon atoms are not satisfied is said to be unsaturated. Such unsaturated molecules are important in the polymerisation i.e., joining together of small molecules into large ones having the same constituents.
No comments:
Post a Comment