
Every gadget we use every day is made of materials that have amazing properties that make them work. However, not every device actually requires the strongest material. There will always be a need to find the strongest substance on earth.
If you think of the world’s strongest material, diamonds are likely to be the first thing that comes to mind.
But is diamond the toughest material?
In a certain sense, you are correct. It is frequently referred to as the world’s toughest natural substance. However, this response is greatly oversimplified, as with most things.
First of all, hardness (and strength) are not the same thing. In certain ways, materials can be exceedingly powerful, but not in others. Some people can better absorb force while others can better withstand scratching. New synthetic materials are also being developed that combine many of these qualities.
However, before starting I would like to explain to you the parameter based on which these materials are arranged in this list. A metric called Power Value (PV) is introduced which is equivalent to the average of tensile strength and density of the material. Based on power value these materials are listed in increasing order (weakest to strongest).
So let’s get started!
These are the 15 strongest materials known to man –
Aerographene

Imagine the strength of graphene mixed with unfathomable lightness. This substance is also known as graphene aerogel. You simply cannot imagine it. Let’s make it even more unbelievable, shall we? Seven times as light as air! This remarkable substance can absorb up to 900 times its own weight in oil and recover fully after more than 90% compression.
There is hope that this substance will be effective in cleaning up oil spills. It doesn’t melt until it reaches a temperature of 1200 °C, making them very strong and resilient materials that can endure pressure hundreds of times their mass.
Applications
- Since its creation, a variety of uses for aerographite have been suggested. These include applications like electrically conductive adhesives, electronics for aviation, satellites, and racing, as well as lightweight batteries for cutting-edge green transportation.
- Additionally, the substance can be used to coat electrically non-conductive objects like plastics to improve their conductivity.
- It serves as a portable contamination absorbent in pollutant control.
In 2013, a team of researchers led by Gao Chao from Zhejiang University made the discovery of aerographene. Earlier, this team was developing graphene-based materials, but these were exclusively 1-D or 2-D compounds. They created the three-dimensional material known as aerographene by first creating freeze-dried carbon nanotube solutions with graphene oxide and then removing the oxygen. Aerographene was a fantastic technology that is quickly gaining popularity while being relatively recent.
Although graphene is created practically anywhere on earth, China, Brazil, and Canada are among the top producers of the material. Naturally, these nations have an advantage in the production of aero graphene.
| Properties | Value |
| Tensile Strength | 0.00016 GPa |
| Elastic Modulus | 0.05 GPa |
| Density | 0.00016 g/cm3 |
| Power Value | 0.00016 |
Darwin’s bark spider’s silk

A spider that was found in the depths of Madagascar’s rainforests constructs the largest webs in the world using silk that is more resilient than any other known biological material. The inch-wide Caerostris Darwini, often known as Darwin’s bark spider, may cover 30-square-foot areas with webs that dangle in midair from 80-foot-long anchor threads.
The spider’s web, which is 25 m wide, is 10 times stronger than kevlar of the same size and the strongest organic substance yet investigated.
What are some potential applications of Darwin’s bark spider silk?
Applications of Spider Silk
- Protective garments against gunfire.
- lightweight clothing that is resistant to wear.
- Ropes, nets, buckled seat belts, and parachutes are all essential safety equipment.
- panels on motor vehicles or boats that are resistant to rust.
- Supports blood vessels that are fragile, such as artificial tendons or ligaments.
In their paper in 2010, Matja Kuntner and Ingi Agnarsson were the first people to formally characterize Caerostris Darwini. The description of the species was completed on November 24, 2009, exactly 150 years after the publication of Charles Darwin’s The Origin of Species. The species’ name is a tribute to the naturalist Charles Darwin.
| Properties | Value |
| Tensile Strength | 1.6 GPa |
| Density | 1.3 g/cm3 |
| Elastic Modulus | 10 GPa |
| Extensibility | 52% |
| Toughness | 354 MJ m3 |
| Power Value | 1.45 |
Limpet teeth
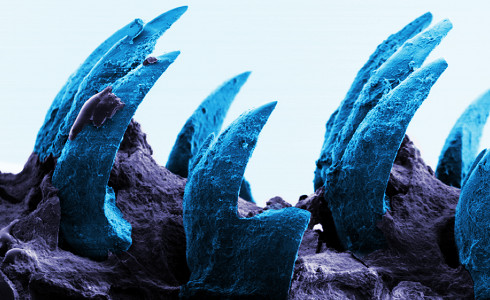
The strongest substance on Limpet, a marine snail, is its teeth, which have a biological basis. It is composed of a mixture of proteins and minerals termed goethite, an iron-based mineral. This substance has a tensile of 4.5 GPa and is almost five times stronger than spider silk. These teeth allow them to pierce rocks and retrieve food from them.
Application
- Limpets scrape food off rocks and into their mouths using a tongue covered in tiny teeth, frequently swallowing small pieces of rock in the process.
- During high tide, limpets use their teeth to hold onto rocks and scrape off algae for nourishment.
- Future automobiles, boats, and even airplanes may incorporate the fiber structure of limpet teeth, according to scientists.
Engineers discovered the strongest biological substance ever tested in the teeth of limpets in 2015. The composite, which the researchers tested in lab samples of very small sizes, is used to make the teeth. They discovered it to be stronger than all but the toughest man-made materials, including spider silk.
These fibers, which are made of the iron-based mineral goethite, are woven into a protein basis in a manner similar to how carbon fibers are used to reinforce plastic. The limpet tooth is nearly as good as the top high-performance carbon fiber materials and is more durable than Kevlar fibers in terms of man-made materials.
| Properties | Value |
| Tensile Strength | 4.5 GPa |
| Elastic Modulus | 140 GPa |
| Density | 0.0009 g /cm3 |
| Vickers hardness | 646 kg⋅m−1⋅s−2 |
| Critical fiber length | 420 – 800 nm |
| Power Value | 2.25 |
Silicon carbide (moissanite)

A hard chemical compound made of silicon and carbon is called silicon carbide, commonly known as carborundum. Since 1893, it has been mass-produced as a powder and crystal for use as an abrasive. It is a semiconductor that naturally occurs as the incredibly uncommon mineral moissanite. It is created by smelting raw materials at a high temperature in a resistive furnace, including quartz sand, petroleum coke, wood chips, and other things.
Application
- Today, silicon carbide elements are used for a variety of processes, including the melting of glass and non-ferrous metals, heat treating of metals, the manufacturing of float glass, the manufacture of ceramics and electronics components, the ignition of gas heater pilot lights, etc.
- For abrasive machining techniques like grinding, sandblasting, and water-jet cutting, silicon carbide powder is used.
- For use in difficult, high-temperature, and corrosive settings, silicon carbide is utilized as a sensing device in the chemical industry, as well as in the turbine or engine testing sectors.
American inventor Edward G. Acheson found silicon carbide in 1891. China (64%) is the world’s biggest producer of silicon carbide, followed by Russia (8.5%) and India (5.2%).
| Properties | Value |
| Tensile Strength | 1.65 GPa |
| Elastic Modulus | 137 GPa |
| Density | 3.21 g/cm3 |
| Specific gravity | 3.218–3.22 |
| Refractive index | 2.654 |
| Luster | Adamantine to metallic |
| Bulk Modulus | 176 GPa |
| Power Value | 2.43 |
Dyneema and UHMWPE

UHMWPE, often known as Dyneema and Spectra, is a type of polymer fiber. Strong polyethylene derivatives make up both of these fibers. These fibers are more durable than Kevlar, another well-known aramid fiber. They outperform steel by approximately 15 times.
The strongest fiber in the world is allegedly Dyneema. The fact that it can stop bullets despite being lighter than water is arguably its most astounding quality.
Applications
- Composite plates in armor, particularly personal armor, and occasionally vehicle armor, utilize derivatives of UHMWPE yarn.
- UHMWPE fibers are used in a variety of civil applications, including cut-resistant gloves, tear-resistant hoses, bow strings, climbing gear, vehicle winching, fishing lines, speargun spear lines, high-performance sails, etc.
- A laminated material called Dyneema Composite Fabric (DCF) is made of two thin transparent polyester membranes and a grid of Dyneema threads. Originally known as “Cuben Fiber,” this material was created to be used in racing boat sails and is incredibly strong for its weight. More lately, it has discovered new uses, most notably in the production of ultralight and light camping gear, including tents and backpacks.
U Sir John Charnley utilized UHMWPE for the first time in a clinical setting in 1962, and by the 1970s, it had become the standard bearing material for total hip and knee replacements.
The top 3producers of the UHMWPE sheet are India, China, and Germany. The world sells the majority of its UHMWPE sheet to India, the United States, and the United Arab Emirates. With 168 shipments, India is the top exporter of UHMWPE sheets, followed by China with 138 and Germany in third place with 74 shipments.
| Properties | Value |
| Tensile Strength | 3.9 GPa |
| Elastic Modulus | 110 GPa |
| Density | 0.98 g/cm3 |
| Tenacity | 3.53 N/tex |
| Maximum Service Temperature | 150 °C (302 °F) |
| Power Value | 2.44 |
Kevlar
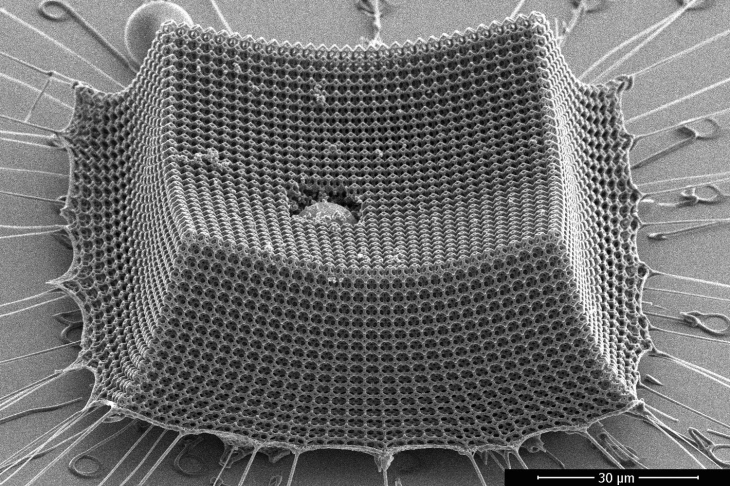
Similar to other aramids like Nomex and Technora, Kevlar is a robust, heat-resistant synthetic fabric. Typically, it is spun into fabric sheets or ropes that can be utilized alone or as a component of composite material parts.
Application
- Due to its high tensile strength-to-weight ratio, which is five times greater than steel, kevlar has a wide range of uses, including protective vests, racing sails, and bicycle tires.
- Along with being used for mooring lines and other underwater uses, it is also employed to create contemporary marching drumheads that can withstand significant impact.
- Typically, it is used to create composites. Kevlar can be used to create hybrid composites by fusing it with other fibers.
When DuPont sought fibers stronger and lighter than steel in 1965, chemist Stephanie Kwolek made the discovery of Kevlar. Kevlar fiber production is actually an oligopoly. Du Pont is the world’s largest manufacturer of para-aramids because it makes Kevlar. Currently, Du Pont produces in three nations: Japan, Northern Ireland, and the United States.
Out of the total manufacturing capacity of 94.7 million lb (4,29,55,162.4 kg) for aramid fibers, these three facilities have a production capacity of 65.9 million lb (2,98,91,712.8 kg). Additionally, Du Pont is responsible for around one-third of global output in Europe and roughly half of the global production in Japan.
| Properties | Value |
| Tensile Strength | 3.6 GPa |
| Elastic Modulus | 76 GPa |
| Density | 1.44 g/cm3 |
| Tenacity | 2.08 N/tex |
| Elongation at Break | 2.4 % |
| Maximum Service Temperature, Air | 149 – 177 °C (300 – 351 °F) |
| Poissons Ratio | 0.36 |
| Power Value | 2.52 |
Metallic Glass

Amorphous metal, which is typically an alloy containing both glass and amorphous structure, is another name for metallic glass. Due to its dual structure, it possesses numerous qualities that are not present in crystalline metal or glass, including good electrical conductivity, high strength, high elasticity, increased wear, and corrosion resistance. Harder than hard tool steel and stronger than steel is metallic glass.
Applications
- Metallic glasses are used to create accurate standard resistance, computer memories, and magnetic resistance sensors due to their high electrical resistance.
- Metallic glasses are ideal for cutting and producing surgical tools due to their great corrosion resistance.
- Because they have strong corrosion resistance, metallic glasses based on chromium and phosphorus are utilized for the inside surfaces of reactor vessels.
The alloy (Au75Si25) created in 1960 at Caltech by W. Klement (Jr.), Willens, and Duwez was the first metallic glass to be documented.
The two countries that produce the most metallic glasses are China and the United States, respectively. Japan ranks third in the world for metallic glass production.
| Properties | Value |
| Tensile Strength | 2 GPa |
| Elastic Modulus | 132 GPa |
| Density | 4.3-5.1 g/cm3 |
| Thickness | 30μm |
| Resistivity | 130Ωμcm |
| Power Value | 3.35 |
Zylon

A synthetic polymer fiber with great tensile strength, rigidity, and flame and heat resistance is called Zylon. This fiber’s exceptional strength is the result of a special chemical bond. A single 1.5 mm fiber strand, in fact, has enough tensile strength to support the weight of a small car, according to Toyoba!
Zylon is utilized in a variety of applications that call for extremely high strength and exceptional thermal stability, much like Kevlar.
Application
- Several of the most well-known goods include tennis racquets, table tennis blades, numerous medical devices, and some martian rovers.
- Zylon is used in many products, including safety gloves, bulletproof jackets, and clothing for firefighters.
- Zylon is used in a variety of industrial applications. Heat-resistant felt made of Zylon is produced for a range of uses. For welding machines, it can be utilized as a cable cover material. Even as a reinforcing component, it can be found in cement.
Manufacturing a polymer and dry-jet wet spinning the liquid polymer into a fibrous material strand produces Zylon, which has an isotropic crystal structure. The fibrous strands can then be wrapped to make yarns, which can then be woven for a variety of fabric types. The fibers can also be divided into staple fibers for use in additional manufacturing procedures.
SRI International created and developed this synthetic polymer material in the 1980s, and Toyobo produced it. Fiber is known as PBO in general usage. Since Toyoba, the firm that began producing Zylon, is based in Japan and has been doing so ever since it seems apparent that Japan is the country that produces the most Zylon.
| Properties | Value |
| Tensile Strength | 5.8 GPa |
| Elastic Modulus | 270 GPa |
| Density | 1.56 g/cm3 |
| Specific gravity | 1.54 |
| Tenacity | 37 cN/dtex |
| Elongation at break | 3.5 % |
| Filament decitex | 1.7 dtex |
| Moisture Regain | 2 % |
| Power Value | 3.68 |
Metallic Microlattice

Metallic microlattice, the world’s lightest metal, is also one of the planet’s lightest construction materials. It is allegedly 100 times lighter than Styrofoam, according to some. It has applications in many engineering sectors because it is a synthetic, porous, and incredibly strong material. It has been cited by Boeing as a material used mostly in the walls, seat frames, and flooring of aircraft.
Application
- lightweight structural designs that can withstand shock, vibration, and noise.
- Numerous potential uses for metallic microlattice technology exist in the fields of automotive and aviation engineering.
- A micro lattice layer serves as the memory device’s crash protection in the flight recorder protection system.
It was originally revealed in November 2011 and was created by a group of scientists from California’s HRL Laboratories in conjunction with researchers from the University of California, Irvine, and Caltech. The prototype samples were created from an alloy of nickel and phosphorous. The microlattice prototype was named one of Popular Mechanics’ 10 World-Changing Innovations in 2012.
| Properties | Value |
| Tensile Strength | 10 GPa |
| Density | 0.0009 g/cm3 |
| hollowness | 99.99% |
| Power Value | 5 |
Diamond

One type of carbon allotrope is diamond. While man-made diamonds can also be created in a lab, this one is found naturally. Each carbon creates four covalent bonds, and the crystal structure is a very small cubic one without any free electrons.
Applications
- Diamonds are utilized in the manufacturing of automobiles and are a crucial component of the automotive industry. Diamond-coated grinding wheels are used to bevel and polish window glass, while diamond saws and drill bits are used to cut and finish automobile bodies and engine components.
- Diamond membranes are translucent, highly enduring, and abrasion and heat-resistant. Diamond windows are used to cover apertures in lasers, vacuum chambers, and x-ray machines. They are manufactured from incredibly thin diamond membranes.
- Because diamonds are unbreakable and even scratch-resistant, they are used to engrave hard stones such as granite and quartz.
Natural diamonds were originally discovered by humans in Indian caves between 4 and 6 B.C., but it had been millions of years since they had been produced.
Around 700 million carats of industrial diamonds are thought to be in reserves worldwide, with the majority being in Australia (31%), the Congo (Kinshasa), Botswana (19%), South Africa (10%), and Russia (6%). Russia (31%), Botswana (20%), and Congo Democratic Republic are the top three diamond producers.
| Properties | Value |
| Tensile Strength | 20 GPa |
| Elastic Modulus | 1050 GPa |
| Density | 3.5–3.53 g/cm3 |
| Bulk modulus | 437.88 GPa |
| Specific gravity | 3.52±0.01 |
| Luster | Adamantine |
| Diaphaneity | Transparent to subtransparent to translucent |
| Refractive index | 2.418 |
| Optical | Isotropic |
| Power Value | 11.75 |
Wurtzite Boron Nitride
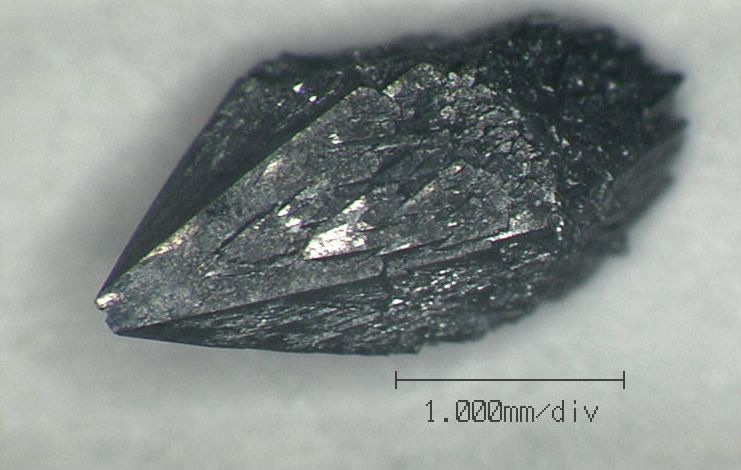
One of the strongest substances in the world is wurtzite boron nitride. It is made up of hexagons. It has a theoretical Tensile Strength of up to 90 GPa, which is comparable to bucky paper. Although for practical purposes it is supposed to have 33 GPa. Different forms of boron nitride have different tensile strengths based on their structure.
It has yet to be demonstrated, though. It is challenging to show because of the restrictions on the availability of w-BN. It is still thought to be a stronger substance than a diamond in the interim.
Applications
- The basic material for sintering coarse grit polycrystalline structure powders, which are then utilized to make conventional cutting and grinding tools, is wurtzite boron nitride with plate-shaped particles.
- It is a very tough material that is used in heat shields, resistance materials, and antiwear additives, among other things.
- Wurtzite boron nitride (w-BN) has recently attracted a lot of interest from both academic and industrial fields due to its superior properties and many potential practical and scientific applications, such as ideal machining/cutting/milling ferrous and carbide materials, especially as an ideal dielectric substrate material for optical, electronic, and 2-D graphene-based devices.
Balmain initially created boron nitride in 1842 by reacting molten boric acid with potassium cyanide (KCN) (H3BO3). Forms like w-BN quickly became common.
Germany, the United States, Japan, China, and other countries are home to the majority of Wurtzite Boron Nitride production.
| Properties | Value |
| Tensile Strength | 33 GPa |
| Elastic Modulus | 400 GPa |
| Density | 3.49 g/cm3 |
| Bulk modulus | 400 GPa |
| Refractive index | 2.05 |
| Knoop hardness | 34 |
| Power Value | 18.25 |
Carbon Nanotube Paper (Bucky Paper)

A carbon nanotube aggregate or carbon nanotube grid paper is used to create buckypaper, a thin sheet. Compared to human hair, the nanotubes are roughly 50,000 times thinner.
It was created initially as a method of handling carbon nanotubes, but it is also being researched and developed into applications by numerous research organizations, showing promise as next-generation electronics and displays, personal armor, and vehicle armor.
Applications
- Protection from fire: Applying a tiny layer of buckypaper increases a material’s fire resistance greatly because the dense, compact layer of carbon nanotubes or carbon fibers effectively reflects heat.
- Microparticles in air or liquid could be captured by buckypaper acting as a filter membrane.
- You can develop biological tissue on buckypaper, like nerve cells. It is possible to electrify or functionalize buckypaper to promote the growth of particular cell types.
In order to prepare samples for various testing, Nobel Laureate Richard Smalley created the first buckypaper in the 1990s by filtering a suspension of nanotubes.
The top three countries for producing carbon nanotube goods are the USA, China, and Turkey, with respective shares of 34%, 32%, and 12%.
| Properties | Value |
| Tensile Strength | 90 GPa |
| Elastic Modulus | 151 GPa |
| Density | 0.50 g/cm3 |
| Electrical conductivity | 0.6 S/m |
| Power Value | 45.25 |
Graphene
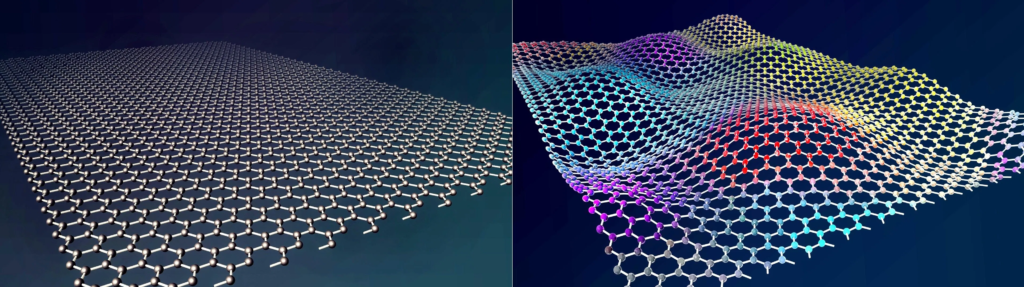
A two-dimensional honeycomb structure called graphene is created when carbon atoms undergo sp2 hybridization. In addition to being the thinnest new material currently known, it has a monolayer sheet structure that is isolated from graphite.
Since the carbon allotrope in graphite contains a lot of double bonds, the name is derived from “graphite” and the suffix -ene.
Applications
There have been significantly more global graphene patent applications since the 2010 Nobel Prize in Physics was given out. Future applications are anticipated in a wide range of industries, including biomedical research, electronics, energy storage, catalysts, sensors, optoelectronic transparent thin films, and super-strength composite materials.
Graphite occurs naturally in three different forms: crystalline flake, amorphous, and lump or vein. Graphite is utilized in a variety of applications depending on its shape.
At The University of Manchester, Professors Andre Geim and Kostya Novoselov made the discovery of graphene in 2004. Although graphene is created practically anywhere on earth, China, Brazil, and Canada are among the top producers of the material. They are the ones who produce the most graphene.
| Properties | Value |
| Tensile Strength | 130 GPa |
| Density | 2.26 g/cm3 |
| Elastic Modulus | 1100 GPa |
| Specific Gravity | 2.09 – 2.23 |
| Colour | Iron black to steel-grey |
| Lustre | Sub-Metallic |
| Transparency | Opaque |
| Power Value | 66.13 |
Lonsdaleite

In regard to its crystal structure, lonsdaleite, also known as a hexagonal diamond, is an allotrope of carbon having a hexagonal lattice as opposed to the cubical lattice of a typical diamond. It occurs naturally in the form of meteorite debris; when meteorites that contain graphite collide with the Earth, the resulting intense heat and stress turn the graphite into a diamond while preserving its hexagonal crystal structure.
Applications
- A common asteroidal impact marker is lonsdaleite, commonly known as a hexagonal diamond.
- Calculations indicate that it has mechanical properties that are superior to diamond and that it is believed to play a key role in the transition of graphite into diamond.
- Lonsdaleite, which has since been utilized as a shock and meteorite impact indicator, was originally attributed to the shock-induced alteration of graphite within the iron meteorite upon impact.
Lonsdaleite, which appears as small crystals paired with regular diamonds in the Canyon Diablo meteorite, was initially discovered in 1967.
Several meteorites, including the Canyon Diablo, Kenna, and Allan Hills 77283 meteorites, include small crystals of lonsdaleite along with diamond. It also naturally occurs in placer deposits of non-bolide diamonds in the Sakha Republic of Russia.
| Properties | Value |
| Tensile Strength | 152 GPa |
| Elastic Modulus | 1325 GPa |
| Density | 3.51 g/cm3 |
| Bulk modulus | 437.09 GPa |
| Specific Gravity | 3.2 |
| Colour | Transparent brownish-yellow, grayish |
| Lustre | Adamantine |
| Refractive index | 2.404 |
| Power Value | 77.75 |
Carbyne
A chain of carbon atoms connected by double or alternating triple and single bonds are called a carbyne. In contrast to graphene, which is like a sheet, it is more like a rope.
The substance with the highest tensile strength to date is carbyne, which has double the tensile strength of graphene. Although carbyne has been found in crushed graphite and interstellar dust, only very brief chains of up to 44 atoms have been successfully synthesized.
Applications
- A high-density, reversible hydrogen storage device is made by adorning a carbyne chain with calcium atoms, which absorb hydrogen molecules.
- Nanoelectronics and photonics both hold great potential for using carbyne’s electronic characteristics.
- Similar to graphene, carbyne is only one atom thick, giving it a very high surface area to mass ratio. The surface area of the electrode controls the energy density in energy storage matrices like batteries and supercapacitors, hence this feature is crucial.
Adolf von Baeyer made the initial discovery of this one-dimensional carbon chain in 1885. He even predicted that carbyne would continue to be elusive because of its extreme reactivity, which would invariably cause its instant annihilation. It is believed that carbyne has superior mechanical characteristics to all other materials.
| Properties | Value |
| Tensile Strength | 251 GPa |
| Elastic Modulus | 4631 GPa |
| Density | 2.178 g/cm3 |
| Specific strength | 7.5 × 107 N·m/kg |
| Power Value | 126.5 |
Top 3 materials in terms of tensile strength
- Carbyne tops the chart with 251 GPa
- Lonsdaleite holds the second position with 152 GPa
- And the third goes to Graphene with 130 GPa.
Top 3 materials with the highest densities
When it comes to density, things are very different from tensile strength. The top three materials in this section would be
- Metallic glass with 4.3-5.1 g/cm3
- Diamond with a value of 3.5–3.53 g/cm3
- Lonsdaleite with the value of 3.51 g/cm3
No comments:
Post a Comment