If we want to measure mass, we can use units like grams. For distance, we use meters. One parameter that has many different units of measurement is pressure. Why are there so many different units for pressure, and how do you convert one to another?
There are so many units of pressure because we can quantify force differently; pressure is force divided by area. The chart below shows the conversion between 1 unit of pressure to another for Pa, atm, bar, psi and mmHg/Torr:
Units, Units Everywhere
Pressure is an important parameter in many situations. Gasoline storage containers often have a pressure gauges (like the one below) for safety reasons, espresso machines require consistent conditions to extract coffee beans, while airplane controls require a certain amount of hydraulic pressure to work.
Click here to read about how pressure gauges work!

Why is pressure measured differently, depending on the country you live in or the field you’re working with? There are so many different units for pressure, many of them unrelated. To understand why, we need to look at the equation for pressure:
Pressure is the result of applying Force spread across a surface Area. It has so many different units because people have found creative ways to consistently define what Force (a close relative of energy) is.
Below is a list of some of the more common ways we measure pressure and how they are used today.
Different Units of Pressure
Pascal (Pa)—The SI Unit
The most common unit of pressure, the Pascal (Pa), was only invented in 1971. Before then, it was known as newtons per square meter (N/m2). Keeping its definition, an international committee named it the Pascal—after Blaise Pascal, a pioneer of fluid dynamics.
Pascal is the unit for pressure in the ‘International System of Units’ or SI. SI units are helpful because each parameter can only have one SI unit, so the entire SI system is standardized.
Within the Pascal group of units, you’ll often see millipascal (1 mPa = 0.001 Pa), kilopascal (1 kPa = 1,000 Pa), megapascal (1 MPa = 1,000,000 Pa) and other multiples of 1000.
1 Pa =
- 9.869 x 10-6 atm
- 10-5 bar
- 1.450 x 10-4 psi
- 7.501 × 10−3 mmHg
Standard Atmosphere (atm)
When you’re standing on Earth’s surface, you can imagine a column of air on top of you reaching upward to the edge of the atmosphere. This column of air pushes down on you because of gravity, with force equal to 1 atmosphere of pressure (101,325 Pa).
The atm unit of pressure is useful in chemistry and physics, where a system under ‘standard’ conditions is defined as being under 1 atm of pressure and with a temperature of 25 °C.
1 atm =
- 101,325 Pa
- 1.01325 bar
- 14.6959 psi
- 760 mmHg
While Standard Atmosphere is helpful in many conditions (where we are close to sea level), the issue is that pressure changes quickly as we increase our altitude, and the air becomes colder and less dense.
The table below shows how 1 atm of pressure changes with altitude.
| Altitude (m) | Temp (°C) | Density (kg/m3) | Pressure (atm) | Pressure (Pa) |
|---|---|---|---|---|
| 0 | 15 | 1.225 | 1.000 | 101,325 |
| 600 | 11 | 1.155 | 0.930 | 94,210 |
| 1200 | 7.1 | 1.088 | 0.864 | 87,510 |
| 1800 | 3.1 | 1.024 | 0.801 | 81,200 |
| 2400 | -0.8 | 0.963 | 0.743 | 75,270 |
| 3000 | -4.8 | 0.905 | 0.688 | 69,690 |
Because of this, plane altimeters take advantage of the changes in pressure at different altitudes. They use atmospheric pressure to determine the height that the plane is flying.

Bar (bar)
Closely related to the Standard Atmosphere is the Bar unit, where 1 bar = 100,000 Pa. Since it is only 1% less than 1 atm (101,325 Pa), bar and atm are often seen in the same contexts.
The bar unit was invented by a Norwegian meteorologist, Vilhelm Bjerknes, presumably because 100,000 is a lot of pascals to discuss the weather with. Bar as a unit of pressure is often seen in gas cylinder pressure gauges.

While 1 atm is the atmospheric pressure at an altitude of 0 m and a temperature of 15 °C, 1 bar of pressure is felt at the height of 111 m at 15 °C.
Today, the use of the bar unit for pressure is internationally discouraged except in Europe, where it is still popular.
1 bar =
- 100,000 Pa
- 0.9869 atm
- 14.5038 psi
- 750.06 mmHg
Pounds per Square Inch (psi)
To get around the problem of changing atmospheric pressure, some people decided to measure absolute pressure by measuring the force (in pounds) that acts on an area of one square inch—psi.
From the conversion table above, you’ll see that 1 atm = 14.6959 psi. This means that if a pressure gauge reads 100 psi, the absolute psi (psia) would be 14.6959 + 100 = 114.6959 psia, which would be the pressure exerted relative to a vacuum (0 psi).
Pressure is often measured in psi for the measurement of gases, particularly in cylinders and tires. An NFL football has a psi of around 13, while a SCUBA tank has a psi of over 2000!
1 psi =
- 6,894.76 Pa
- 7.031 x 10-2 atm
- 6.895 x 10-2 bar
- 51.7149 mmHg
Millimeters of Mercury (mmHg) and Torr
The very first method for measuring pressure was to use a device called a manometer, a U-shaped tube filled with mercury.
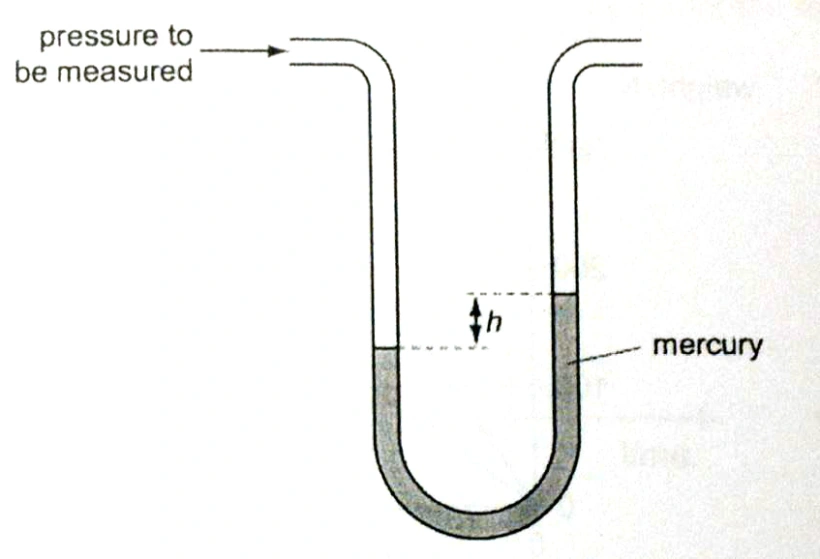
The pressure of gas could be calculated by opening its container so that the gas fills the left side of the manometer and pushes the mercury downward. Then, the change in height (h) of mercury on the right side was measured.
We can therefore describe pressure by how much the height changes (in millimeters) of mercury—mmHg. mmHg and Torr are interchangeable because the original definition of Torr is the pressure required to move 1 millimeter of mercury.
1 mmHg =
- 133.322 Pa
- 1.316 x 10-3 atm
- 1.333 x 10-3 bar
- 1.935 x 10-2 psi
Because mercury is toxic, we don’t use manometers such as these anymore. Today, mmHg is still used in medical settings, though mercury manometers have been replaced by calibrated valves in sphygmomanometers.

Blood pressure is often measured in millimeters of mercury values, where systole and diastole are ideally at 120/80 mmHg.
Aside from the ones mentioned above, other methods to measure pressure are still used, though they are slowly being phased out. Hopefully, standardizing all units of pressure to Pascals (and its multiples of 1,000) will help reduce all the confusion and conversion around this topic!
No comments:
Post a Comment