What is Quenching?

On TV, you’ve probably seen bladesmiths dunk a red-hot sword into water or oil. This is called “quenching” (don’t feel bad if you thought this was called “tempering,” that is a common mistake). Quenching is an ancient method of rearranging the atomic structure of a material.
Quenching is the process of rapidly cooling a material (usually a metal) in order to obtain desirable mechanical properties like increased strength and hardness. Most people think quenching is just dunking red-hot steel into a bucket of water, but materials scientists can quench in water, oil, liquid nitrogen, or even air.
Quenching is an important processing step for many materials. Quenching can refine crystal grains or manipulate phase changes. One really important phase transformation is martensite to austenite in steel, which I am going to discuss in-depth later.
To determine the effects that quenching can have on a metal, materials scientists may use Jominy tests or time-temperature-transformation (TTT) curves.
We’ll get to these technical terms later, but first let’s look at all the cool things you can do with quenching.
Outline
- What Is the Purpose of Quenching?
- What Is the History of Quenching?
- What Are Quenching Media?
- What Happens During the Quenching of Steel?
- What Are the Characteristics of a Martensitic Phase Transformation?
- What Are the Key Features of Martensite?
- What Is Retained Austenite?
- How Does Carbon Content Affect the Hardness of Quenched Steel?
- What Are the Steps to Quenching?
- How Quickly Do We Need to Quench to Achieve Martensite (What is a TTT Curve?)
- Final Thoughts
- References and Further Reading
What Is the Purpose of Quenching?
When most people think of quenching, they probably picture a blacksmith dropping a red-hot piece of steel into a bucket of water. Quenching is much broader than this, but let’s start with this example and explore what quenching can do in steel.
The first thing that quenching can do is to refine grains.
If you didn’t know, metals are crystals. Unlike the crystals you might normally think of, like gemstones, metals are “polycrystalline.” That means that metals are composed of many, many tiny crystals. We call each of these small crystals a “grain.”
If it’s blowing your mind that metals are actually crystals, open the collapsible text below for a brief introduction to crystallography.
Crash Course in Metallurgical Crystallography
A crystal is any material that has a repeating atomic structure. Diamond, salt, ice, sugar, and steel are all examples of crystals. Actually, most materials are crystals!
It turns out that a repeating pattern is a very stable way for atoms to arrange themselves. The three most common crystal structures are Body-Centered Cubic (BCC), Face-Centered Cubic (FCC) and Hexagonal Close-Packed (HCP).
The way that the atoms are arranged can have a huge effect on material properties–just look at diamond vs graphite! A major part of materials science is influencing material properties by changing the crystal structure.
When the layperson talks about a crystal, he or she usually means something like a gemstone–diamond, ruby, sapphire, or salt. These are a special type of crystal: a single crystal. Single crystals have an uninterrupted pattern of atoms through the entire material.
More commonly, materials such as metals are polycrystals. These materials still have a repeating structure, but that structure is broken up into different grains.
When the phase solidifies, it starts as just a few atoms. These atoms cluster together, then grow. All the atoms that join this cluster will have the same orientation, and they will form a grain. At some point, the grain will run into another grain.
If the phase solidified more slowly, there would be fewer initial grains, and each grain would be larger. if the phase solidified more quickly, there would be many initial grains and each grain would be smaller.
In general, smaller grains means a stronger, less ductile metal. I hope I have illustrated this with my homemade animations, but if you want to look this up yourself, it’s called the Hall-Petch Effect.
Now, in my explanation I may have implied something which is not true. This model of grain nucleation and growth looks very nice when you are creating a solid phase from a liquid, but that’s not what quenching normally is.
For example in steel, quenching takes us from one solid phase to another solid phase. This still leads to grain refinement, but it also has much more important implications. I’m about to throw some new vocabulary words at you, hold on.
Steel starts in a phase called “ferrite.” As you heat the steel, it changes to a new phase, “austenite.” Austenite can dissolve more carbon than ferrite–which is fine. If you cool austenite you will get ferrite again–as long as you cool the steel slowly enough that the carbon has time to leave the metal.
On the other hand, if you cool the steel quickly, you will get a new phase: “martensite.” Martensite is very hard and very stressed. Martensite is basically ferrite that has too much carbon trapped inside. All this carbon distorts the crystal, so we call the new phase martensite.
This distorted crystal is very hard, but quite brittle. Swords, knives, and other tools are made of martensitic steel to obtain high strength.
But if you look at a phase diagram, you won’t see martensite. Martensite is not a thermodynamically stable phase–but you can still reach it in your final product. The diagram that tells you about austenite is called a time-temperature-transformation diagram, or TTT curve. We’ll talk about the TTT diagram later.
So, we’ve learned that quenching can make grains smaller, and it can create a stressed, stronger phase. Both of these effects will make the steel stronger but less ductile.
In some cases, quenching can make a material more ductile, and softer. In the case of most steels, you saw that the speed of quenching allowed you to get a metastable martensite, instead of the thermodynamically favored ferrite.
However, in other materials (or some unique steels), quenching can let you avoid a strengthening or embrittling phase.
Take a look at superalloys. Ni-based superalloys have a very nice phase called γ’ (pronounced “gamma-prime”). This is the thermodynamically stable phase, but there are other “bad” phases called TCPs. These technically make superalloys stronger, but they also make superalloys much more brittle/less ductile.
Usually, materials scientists fine-tune strength (how much force a material can survive) vs. ductility (how much deformation a material can survive). TCPs are slightly stronger but much more brittle, so γ’ is better than TCPs in 99% of applications.
When you make a superalloy, if you cool it slowly, the TCPs may form. These are often stable at higher temperatures than γ’. Unfortunately, once the superalloy is cold enough that γ’ is more stable than TCPs, diffusion is quite slow and the TCP will last forever.
But if you quench the superalloy, you skip this temperature range where TCPs are stable and you only get γ’.
Quenching can also be used for thermal tempering in glass. Yes, the terminology is weird because we usually use the word “tempering’ to refer to making a metal weaker after quenching, but thermal tempering is a way to make glass stronger.
Thermal tempering is a key feature of Prince Rupert’s drops, so if you want to learn more about the process, I recommend you check out this article.
What Is the History of Quenching?
Quenching has existed long before scientists knew how it worked. Don’t feel bad if you don’t understand quenching either!
Old blacksmiths and bladesmiths learned techniques by trial-and-error, and kept their techniques guarded closely. Some legends claim that great bladesmiths would quench swords into the bodies of slaves (actually it’s possible that this would have worked, or at least that blood might sometimes be better than water, see the section on quenching media below).
Quenching a sword creates huge stresses in the sword. Often, imperfect swords would break. Different quenching methods or media could be used to reduce the likelihood of the steel shattering, but this level of metallurgy was surrounded by mysticism until fairly recently.
Perhaps the first written reference to quenching is in Homer’s The Odyssey “ . . . a blacksmith plunges an axe or hatchet into cold water to temper it—for it is this that gives strength to the iron . . .” And yes, the translator I quoted confused quench/temper.
Quenching has been an important aspect of swordmaking for centuries, but Japanese swordsmiths had perhaps the most sophisticated quenching technique.
Japanese swords were made by quenching at a gradient. They coated their blades with clay so that the back of the sword quenched more slowly, so it had more tough ferrite. The edge of the sword would be pure, hard martensite while the core of the blade was tougher and more ductile.
What Are Quenching Media?
As you’ll learn below in our section on TTT curves, different quenching rates can produce different results. Different metals can survive different quenches without cracking.
In some steels, you may want a different ratio of martensite to ferrite. And of course, non-steel metals behave much differently than steels and require their own quenching methods. Most of the time, quenching is simply a function of how fast you cool the material.
Generally, the only way to control cooling speed is with the quenching medium (that’s the stuff in the bucket). In theory you could change the temperature of the quenching medium, but you already have a hot metal, so a few degrees change in the water temperature doesn’t really matter. On the other hand, differences in specific heat or boiling point can make a large difference in cooling rate.
The most common quenching media are water, brine (salt water), oil, liquid nitrogen, and air. Each of these media has different advantages and disadvantages.
Water
Water is one of the most common quenching media, because it’s easy to get and results in a fast quench. Water isn’t flammable and has a large specific heat and heat of vaporization, so it cools the material quickly when the water boils. However, the bubbles from boiling reduces the thermal conductivity, ultimately leading to a slower quench. (This layer of insulation caused by gas bubbles is called the Leidenfrost effect).
Brine
Brine is just water with salt added. It has many of the same advantages as water, but the salt increases the boiling point of the water, resulting in fewer bubbles from boiling and an even faster quench rate. One downside of a brine quench is that salt can sometimes corrode or otherwise react with some alloys.
Regarding the legends about quenching swords in blood: since blood is also water with electrolytes (salts) dissolved in it, from a quenching standpoint blood is similar to a weak brine (urine would be a more brine-like body fluid though). Blood also has a lot of organic compounds which might coagulate and stick to the blade, reducing the Leidenfrost effect. It’s also possible that these carbon molecules could react to form a small amount of carbides on the surface, although I doubt that would be noticeable. In short, yes it’s possible that quenching in blood could produce a different/more desirable quench than water.
Oil
There are several types of oils, but they all have a lower specific heat than water, resulting in a slower quench. I’ve seen amateur bladesmiths use motor oil, vegetable oil, peanut oil–even frying oil recycled from some students’ favorite fried chicken restaurant!
Oil is a good medium-speed quench and can help avoid cracking. One downside of an oil quench is that the surface of the oil may catch on fire, so you need to be very careful when performing an oil quench.
Liquid Nitrogen
Liquid nitrogen is actually slower-quenching than water, because the nitrogen turns to gas (low thermal conductivity) and has a lower heat capacity and heat of vaporization. However, liquid nitrogen ultimately results in a cooler final quench than other media, which is needed in some steel alloys. For example, many stainless steels don’t precipitate martensite until very low temperatures that water can’t reach.
Air
Generally, an air quench is done by blowing cool air over the sample very quickly. Air quenches are often used in industrial settings because air is very cheap, and by controlling the air speed at different parts of the product, you can have different quench rates at different spots. Air quenching is typically the slowest quenching medium.
Another kind of air quench is just letting a part cool in still air. I usually call this “air cooled” instead of air quenched, but there are some alloys that can achieve a quenched microstructure even at very slow cooling rates–in this situation, this “air quenched” terminology fits.
What Happens During the Quenching of Steel?
Here is a phase diagram of steel. The x-axis shows the percent of carbon, and the y-axis shows the temperature.
When quenching steel, we first need to heat it past the austenitizing temperature. In hypoeutectoid steel (left of the eutectic point) the steel will have the austenite phase. In hypereutectoid steel (right of the eutectic point) the steel will have two phases: austenite and cementite.
To get martensite, the steel will need to change phases when while it’s quenched. Below the austenitizing temperature, nothing will happen when it’s quenched.
Austenite, the Face-Centered Cubic (FCC) form of iron which is usually represented by γ, can dissolve more carbon atoms than ferrite (the Body-Centered Cubic (BCC) form of iron, represented by α). Austenite can dissolve 2% carbon, while ferrite can dissolve 0.025% carbon. This is because of the size and number of interstitial sites in FCC vs BCC structures (you can read more about BCC and FCC crystal structures in this aricle).
When we quench the steel, it cools rapidly and wants to transform from BCC austenite to FCC ferrite. However, the cooling rate is too fast for the carbon atoms to move out of the way, so they essentially get trapped in the FCC phase.
FCC iron with carbon trapped in it is no longer called ferrite: now this is martensite!
Martensite is a supersaturated solution of carbon, and it distorts the body-centered cubic lattice into a body-centered tetragonal lattice.
Depending on the steel composition and quenching rates, the final product may also have ferrite or retained austenite.
What Are the Characteristics of a Martensitic Phase Transformation?
A martensitic phase transformation is also called a “military-order” phase transformation or a “displacive” phase transformation.
These kinds of phase transformations do not involve diffusion. All of the atoms simultaneously shift in the same direction. The martensite typically forms a structure that looks like plates or needles. These are called laths.
As the martensite forms, it creates internal stresses in the steel. These stresses suppress further phase transformation. Remember that phase diagrams actually have 3 axes: composition, temperature, and pressure. In most materials science phase diagrams we only graph composition and temperature because we assume that the pressure is just the atmospheric pressure, but internal stresses act the same as an external pressure.
Newly formed maretniste puts compressive stress on the remaining austenite. This is what causes “retained” austenite. There is almost always some retained austenite, so 100% of the steel is never changing to pure martensite.
Austenite is denser than martensite, so there is a volume increase after quenching (this is why Japanese swords are curved: there is more martensite at the edge, so that part of the blade expands more than the rest, leading to a curved appearance).
Large pieces of steel may even crack when quenched, due to the internal stress caused by martensitic volume expansion. This phenomenon is an especially important issue if the carbon content is greater than 0.5 wt%.
If the steel is quenched too slowly to achieve martensite, you will end up with bainite or pearlite. Bainite occurs when the carbon atoms are able to partially diffuse out of the lattice. Pearlite occurs when full diffusion is achieved.
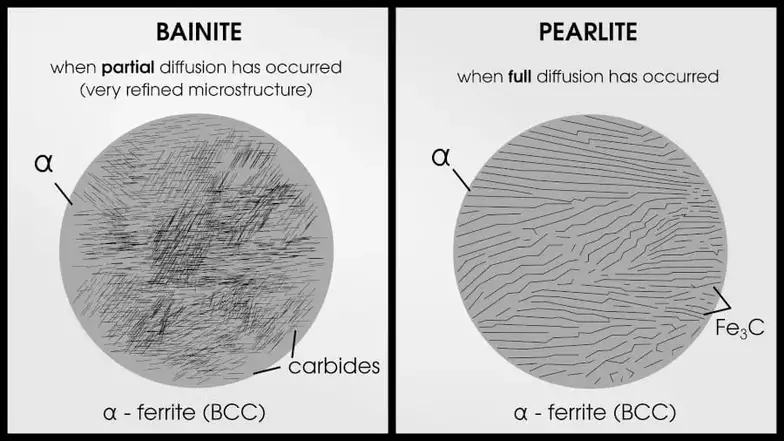
What Are the Key Features of Martensite?
Martensite is the end product of conventional quenching on steel. It is a supersaturated solid solution of carbon in a body-centered tetragonal (BCT) crystal structure. BCT is essentially just BCC, but elongated in one direction. Martensite is BCT because the carbon atoms sit in interstitial sites, but since the carbon atoms are larger than regular BCC interstitial sites, the lattice needs to distort.
Martensite is a metastable phase, which means it is not predicted by thermodynamics and does not appear on any phase diagram. Another name for a metastable phase is a non-equilibrium structure. Given enough time, martensite would eventually decompose, but this would take thousands of years at room temperature.
If martensite is heated, however, the iron and carbon atoms would get enough energy to increase diffusion rates, allowing the atoms to rearrange into the more stable ferrite phase.
Martensite is very hard and brittle. In fact, quenched martensite is too brittle to be used in most engineering applications. Usually, after quenching, steel is tempered. Tempering is a process that reheats steel (below the austenitizing temperature) and cools slowly, in order to reduce some internal stresses.
What Is Retained Austenite?
Retained austenite is actually a stable (well, sometimes metastable) phase. However, it doesn’t show up on the phase diagram at room temperature because the regular phase diagram assumes standard atmospheric pressure.
As pressure increases due to internal stress from martensite volume expansion, austenite becomes more likely. You can see the phase diagram above, for pure iron, shows that increasing pressure prefers austenite. With carbon additions, austenite is stable at even lower temperatures. If austenite is stable all the way until room temperature (this depends on the steel composition), it will be an equilibrium phase. However, even if the retained austenite is stable at high pressure and a few hundred degrees C, in many cases that will allow the metastable retained austenite to persist indefinitely because of slow diffusion rates at lower temperatures.
Often, retained austenite is an undesirable microstructural feature because it is much softer than martensite. As carbon content increases, retained austenite is increasingly likely.
One easy way to test for retained austenite/martensite is to use X-Ray Diffraction (XRD). Since martensite is BCT and austenite is FCC, their different lattice parameters will easily show up on XRD. Integrating over the curves can even provide quantitative values for the fraction of martensite vs retained austenite.
How Does Carbon Content Affect the Hardness of Quenched Steel?
Generally, more carbon leads to harder, more brittle steel. However, more carbon also leads to more retained austenite. Additionally,changes in carbon percent can change the martensite lath shape and increase microcracking.
Past a certain point, additional carbon weakens the steel, as shown in this paper from Litwinchuk et al. In unalloyed carbon steel, maximum hardness from quenching in brine is achieved at around 1 at.% carbon.
What Are the Steps to Quenching?
For reference, here’s the relevant portion of the iron-carbon phase diagram.
First, heat the alloy to 30-50°C higher than the critical temperature. This region is labelled in the diagram above. We don’t want to stay at this temperature for long, because it might cause grain growth.
If you are working on an alloy that is sensitive to oxidation, you may want to heat the alloy in a vacuum. There are furnaces that can heat while under vacuum, but a simpler (small scale) method is to encapsulate your alloy in a quartz tube that has been vacuumed or filled with inert gas, like argon.

The alloy needs to be cooled rapidly. The main way to control the cooling rate is by using a different quenching media. Salt water is usually the fastest practical quenching media. Liquid nitrogen is a relatively slow quenching media because of its low thermal conductivity and specific heat.
If the alloy is cooled too rapidly it may crack. If it’s cooled too slowly, you may not get much of the metastable phase. The best way to determine the optimal quenching speed for a material is to use a Time-Temperature-Transformation (TTT) chart.
How Quickly Do We Need to Quench to Achieve Martensite (What is a TTT Curve?)
A Time-Temperature-Transformation (TTT) diagram is a diagram that shows what phases–including metastable phases–will be present at a certain cooling rate.
Temperature is graphed on the y-axis and time is graphed on the x-axis. Different phases appear, usually in a feature dubbed the “nose.”
The bottom horizontal line is when martensite will precipitate. If you quench fast enough that you miss the nose (red line), you will not precipitate other phases besides martensite. If you do a slower quench, shown by the blue line, you will get other phases such as ferrite and cementite (we call the mix of ferrite and cementite “pearlite.”)
The purple line shows the minimum quench rate required to only precipitate martensite. In this case, that means quenching 500°C in 5 seconds. Quenching any faster than that will not make a difference in the phase fractions present, but faster quenching may result in excessive internal stresses and cracking.
If steel is not quenched fast enough, it may result in bainite or pearlite formation (these are not necessarily bad phases–they are weaker but tougher than martensite).
Final Thoughts
Quenching is one of the most important tools for engineering alloys, especially steels. Quenching is done by heating the metal and rapidly cooling it in a quenching medium such as water or oil.
Proper quenching can precisely control the final microstructure and phases present in the alloy.
No comments:
Post a Comment