What is Sensible heat?
Sensible heat is the heat exchanged by the object that causes a change in temperature of the object without changing its phase.
Hence sensible heating affects the system by changing its temperature. As the name says, we can sense the changes happening during the sensible heating.
Example:- If you start heating to the water kept in a container, then in this case you can sense the temperature changes just by dipping your fingers in the water. As time-lapse, you can feel the rise in temperature, so this process is known as sensible heating.
The unit of the sensible heat in the SI system is the joule (J) and in the FPS system is Btu.
Sensible heat diagram:
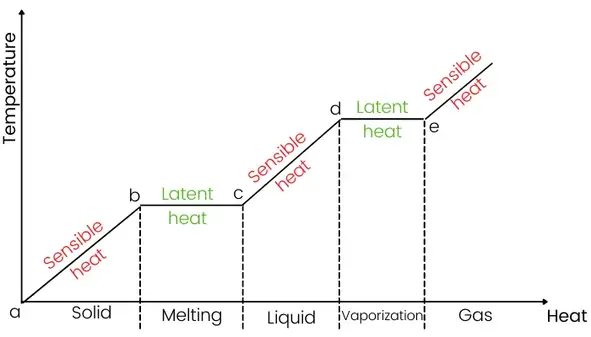
In the above graph, the region a-b, region c-d, and region after e, show the sensible heating process.
Sensible heat explanation:
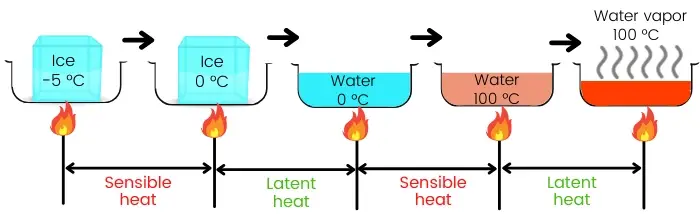
The above figure shows the heating of the ice at -5°C to convert it into water vapors at 100°C.
The complete process consists of a combination of sensible heating and latent heating.
Here ice from -5°C is heated to raise its temperature up to 0°C. Hence the heat supplied is known as Sensible heat.
Again water from 0°C is heated to raise its temperature up to 100°C, hence in this case also the heat supplied is known as Sensible heat.
Sensible heat equation:
The amount of sensible heat supplied or rejected by the substance is given by,
QSensitive = mC(TFinal – TInitial)`
Where,
m = Mass of substance
C = Specific heat of a substance
TFinal = Final temperature
TInitial = Initial temperature
Sensible heat example:
10 Kg of water inside the container at the atmospheric pressure and 25°C is heated to the 80°C. Find the amount of sensible heat required for the heating of the water if Cp(water) = 4.186 KJ/Kg.
Given:
T1 = 25°C
T2 = 80°C
Cp = 4.186 KJ/Kg
m = 10 Kg
Solution:-
The amount of sensible heat required for the heating of water is given by,
Qsensible = m.Cp.[T2 – T1]
Qsensible = 10 x 4.186 x [80 – 25]
Qsensible = 2302.3 KJ
FAQ
Why is sensible heating important?
Sensible heating helps to increase or decrease the temperature of the system without changing the phase of the system.
What is sensible heat in air conditioning?
In the case of air conditioning, the amount of heat required to raise or lower the temperature of the air in the cabin is known as sensible heat.
No comments:
Post a Comment