The very fine suspended and colloidal particles in water do not settle under gravity In plain sedimentation thanks. Such particles can however, be removed by increasing their size and mass i.e., by changing them into flocculated particles.
COAGULATION AND FLOCCULATION
The very fine suspended and colloidal particles in water do not settle under gravity In plain sedimentation thanks. Such particles can however, be removed by increasing their size and mass i.e., by changing them into flocculated particles. To achieve this chemicals called coagulants. are added to water which forms gelatinous precipitate called 'floc'. Most of the colloidal particles in water are negatively charged. The coagulants (positive charge) neutralize the negatively charged colloidal particles and allow them to coagulate (agglomerate) and form a gelatinous precipitate called 'floc' to which more and more particles get attracted and absorbed, forming bigger sized flocculated particles. The flocculated particles due to increase in size and mass readily settles in the sedimentation tanks. The chemically assisted sedimentation process also known as clarification comprises of following three stages:
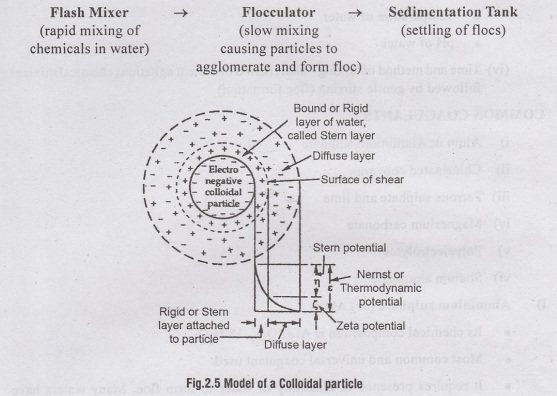
(i) Addition of measured quantities of chemicals (coagulants) to water and thorough mixing is done in a Flash Mixer.
(ii) Formation of precipitate which coagulates and forms floc which happens in a Flocculator.
(iii) Settling of flocs in a Sedimentation Tank.
Coagulation is the first stage which refers to the formation of precipitate and destabilisation of charged colloidal particles.
Flocculation - is the second stage which refers to the slow mixing technique promoting agglomeration of stabilised particles.
Factors affecting coagulation:
(i) Type of coagulant,
(ii) Quantity or dose of coagulant
(iii) Characteristics of water.
• Type and quantity of suspended matter.
• Temperature of water
• pH of water
(iv) Time and method of mixing – short period of violent agitation (chemical mixing) followed by gentle stirring (floc formation)
COMMON COAGULANTS
i) Alum or Aluminium sulphate
ii) Chlorinated copperas
iii) Ferrous sulphate and lime
iv) Magnesium carbonate
v) Polyelectrolytes
vi) Sodium aluminate
i) Aluminium sulphate (or) Alum
• Its chemical composition is Al2(SO4)3 18 H2O
• Most common and universal coagulant used.
• It requires presence of alkalinity in water to form floc. Many waters have bicarbonate alkalinity.
Alum dissolved in water, hydrolyze into Aluminium Hydroxide (insoluble – floc)
Al2(SO4)3 18H2O + 3Ca(HCO3)2=2 Al(OH)3↓ + 3CaSO4 +18H2O + 6CO2↑ The Aluminium Hydroxide floc so formed is insoluble in water.
Limitations of using Alum :
(i) CO2 formation leads to corrosiveness in water.
(ii) Calcium sulphate formed causes permanent hardness in water.
If water is not naturally alkaline, lime CaO or hydrated lime Ca(OH)2 is added to
Al2(SO4)3. 18H2O + 3Ca(OH)2 = 2AI(OH)3 + 3CaSO4 + 18H2
Sodium carbonate or soda ash can also be added to increase alkalinity.
Al2(SO4)3. 18H2O+ 3Na2CO2 = 2A/(OH)2 + 3Na2SO4+3CO2+18H2
Soda ash does not cause hardness, but it is expensive than lime and is used less. Alum is effective at pH 6.50 to 8.50
Alum Dosage - 10 to 30 mg/litre of water. Its dosage depends upon turbidity, colour, taste, pH and temperature.
Alum reduces turbidity, taste and odour. It produces crystal clear water. It is cheap and commonly used. But it is difficult to dewater the sludge formed.
ii) Chlorinated copperas
Hydrated ferrous sulphate is called copperas (FeSO4. 7H2O)
• It has high solubility.
• It is used after oxidation to ferric sulphate [Fe2(SO4)3] and ferric chloride (FeCl3) by mixing with feed from a chlorinator. The ferric sulphate and ferric chloride are called chlorinated copperas.
6FeSO4 .7H2O + 3Cl2 = 2Fe2(SO4)3 + 2FeCl3 + 7H2O
Chlorinated copperas form ferric hydroxide floc.
Fe2(SO4)3 + 3Ca(OH)2 = 3CaSO4 + 2Fe(OH)3
2FeCl3 + 3Ca(OH)2 = 3CaCl2 + 2Fe(OH)3
They are effective in removing colour also.
Ferric chloride (ferrichlor) is effective over pH 3.5 to 6.5 and above 8.5
Ferric Sulphate (Ferrisul) is effective over pH 4 to 7 and above 9.
iii) Ferrous Sulphate (Copperas) and Lime
Lime is added with ferrous sulphate to increase the reaction rate.
FeSO4 7H2O + Ca(OH)2 = Fe(OH)2 + CaSO4 + 7H2O
Ferrous hydroxide Fe(OH), - floc formed is soon oxidised by dissolved oxygen in water and ferric hydroxide Fe(OH), is formed.
4Fe(OH)2+O2+ 2H2O = 4Fe(OH)3
The ferric hydroxide is a heavier floc.
Ferrous sulphate is effective at pH range above 8.5
iv) Magnesium Carbonate and Lime
When Magnesium Carbonate and Lime are dissolved in water, magnesium hydroxide and calcium carbonate are formed. Both Mg(OH), and CaCO3 are soluble in water resulting in formation of sludge which is a slurry.
MgCO3 + Ca(OH)2 Mg(OH)2 + CaCO3
Due to slurry formation, it is not commonly used. However, it removes organic colour, iron and manganese.
v) Polyelectrolytes
Polyelectrolytes are high molecular weight water-soluble polymers.
They are anionic, cationic or non-ionic. The dosage is 1 ppm.
Cationic polyelectrolytes are available under trade names Floccal N, Magnifloc972, Mogu1980 and are used independently.
Other polyelectrolytes are used as coagulant aids with alum in order to reduce the amount of primary coagulant required.
vi) Sodium Aluminate
Sodium Aluminate Na2Al2O4 is alkaline and used less due to high cost. It removes both temporary and permanent hardness.
The effective pH range is 6 to 8.50. Its reaction with Calcium and Magnesium salts are as under:
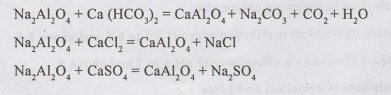
Comparison of Alum and Iron salts (as coagulants)
(i) Iron salts produce heavy floc due to which more suspended matter is removed than with alum.
(ii) Iron salts are good oxidising agents and remove H2S and its corresponding taste and odour.
(iii) Iron salts are effective over wide pH range.
(iv) Iron salts cause staining and growth of iron bacteria in distribution system.
(v) Iron salts impart more corrosiveness to water than alum.
(vi) Skilled suspension is required for handling and storing of iron salts, as they are corrosive. No such supervision is required for alum.
(vii) The time required for floc formation and settling using iron salts is much less.
(viii) Formation of mud balls with iron salts is less compared with alum.
Alum is preferred as coagulant for treating raw waters
Problem 2.1:
Determine the quantity of alum required in order to treat 13 million litres of water per day at a treatment plant, where 12 ppm of alum dose is required. Also determine the amount of CO2 gas which will be released per litre of water treated.
Solution:
Quantity of water to be treated = 13 × 106 litres/day
Alum dose required = 12 ppm = 12mg/1
Amount of alum required per day = (13 x 106) x 12 mg = 156 kg.
Chemical reaction involved is given by:

No comments:
Post a Comment