What are the Difference between Heat and Thermal Energy?
Contents:
Heat:
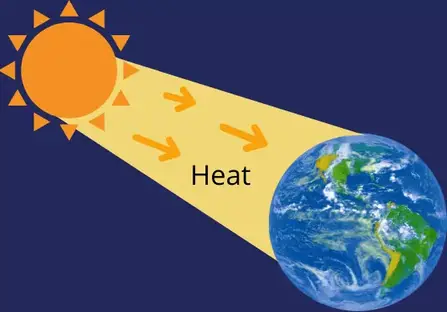
Heat is the transfer of energy from one system to another system because of the presence of temperature differences between these two systems.
In simple words, heat is the energy in flow from the hot body to the cold body. The direction of this energy flow is always from the hotter object to the cold object.
Example: Heat transfer from the sun to earth.
Thermal energy:

Thermal energy is the kinetic energy associated with the motion of the molecules in a system or object which is responsible for the temperature of the object.
Thermal energy is the property of the system that describes the particular state of the system.
As the temperature increases, the kinetic energy of the molecules also increases which results in an increase in thermal energy. Similarly, as temperature decreases, the thermal energy of the system also decreases.
Example: Thermal energy associated with sun because of the high temperature.
What is the difference between heat and thermal energy?
| Sr. no. | Heat | Thermal energy |
|---|---|---|
| 1] | Heat is the transfer of energy between two systems with different temperatures. | Thermal energy is the kinetic energy of the molecules of the system because of the temperature of the system. |
| 2] | The heat becomes internal energy when it enters into the boundary of the system. | The thermal energy becomes heat when it leaves the boundary of the system. |
| 3] | Heat is the process variable. | Thermal energy is the property of the system. |
| 4] | The term heat is used to describe the process or path. | The term thermal energy is used to describe the state of the system. |
| 5] | The heat is a result of the temperature difference between two objects. | Thermal energy is responsible for the system’s temperature. |
No comments:
Post a Comment